Is pf3 polar or nonpolar
Skip to main content.
Post by ishaa Diwakar 4E » Thu Nov 14, am. Post by Jacob Motawakel » Sun Nov 17, am. Post by stephaniekim2K » Sun Nov 17, am. Laurence Lavelle Skip to content. Quick links. Email Link. Polar vs.
Is pf3 polar or nonpolar
Wiki User. PH3 is a non-polar covalent molecule. This is somehow confusing because, when you draw out the Lewis diagram, you will observe a lone pair on the P atom. However, if the electronegativity difference does not have a polar bond, then no matter what happens, it will always be non polar. In this case, the EN is 0. To decide whether a molecule is polar or non-polar , first draw the Lewis diagram. Then calculate the electronegativity. If the electronegativity is non polar, then no matter it has lone pairs or it has more than one group around the central atom, it will always be non polar. In this case, PH3 is non polar because of it has a non polar bond indicated by the electronegativity even though it has lone pairs. But if the bond is polar, then you might have a chance to get a polar molecule depending on either of these 2 factors :. Even if it doesn't have a other groups around the central atom, it's still polar because it's polar if you just satisfy either of the 2 conditions.
Bohr Equation. Previous problem.
Q: Which of the following sets of atoms will NOT form a strong hydrogen bond? Explain your answer a. A: Hydrogen bonding: The hydrogen atom attached with the electronegative atom like fluorine, oxygen, or…. A: The molecule that shows dipole moment is known as polar molecule and bond is known as polar bond. Q: Is HCN polar. A: Definition of polar, Any compound or molecule is polar or not, it depends on the electronegativity….
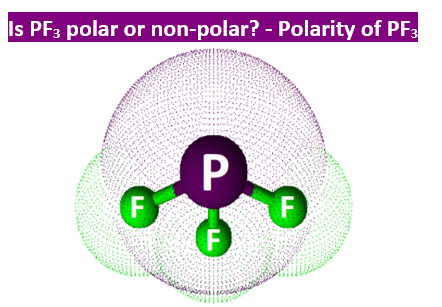
Phosphorus trifluoride is a chemical compound with the chemical formula of PF3. PF3 is known as a ligand in metal complexes, and it has a high level of toxicity in nature. To answer your question if Phosphorus Trifluoride is a polar or nonpolar molecule, our team did thorough research to provide you with all the information about the polarity of this compound. PF3 is a polar molecule. Due to the one lone pair present in the central atom Phosphorus, it generates repulsion. Nonpolar molecules have zero dipole moment, while polar molecules have greater than zero dipole moments.
Is pf3 polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity?
Gucci blue leaks
Gamma Emission. Lewis Dot Structures: Ions. Polar Bonds, Polarity and Intermolecular Forces. Osmotic Pressure. Stephen Stoker. Select the IMF s that it can participate in. Gases 3h 54m. Naming Cyclic Alkanes. Guided course. General Chemistry Q: Which will have the lowest Bp? Significant Figures. Simple Cubic Unit Cell.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Problem 5. O shape of molecules O size of… A: Dispersion forces are the weak intermolecular forces which exist between non-polar molecules like He…. Cl2O Problem 5. ClO3 Problem 5. Trending Questions. SO3 b. Limiting Reagent. Equatorial and Axial Positions. Peroxide and Superoxide Reactions. A: Bond angle is the angle between two adjacent bonds. Is PO4 3- polar or nonpolar? Third Law of Thermodynamics. Arrange the given linear molecules in order of decreasing dipole moment.

I can not take part now in discussion - it is very occupied. I will be free - I will necessarily express the opinion.
I consider, that you are mistaken. Let's discuss it. Write to me in PM.
I will know, many thanks for the information.