Is so2 polar
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two is so2 polar take 6 lone pairs, and the remaining one goes to the sulfur:, is so2 polar.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth. Much like the earth, molecules can have polar regions, but these polar regions are positive and negative in nature.
Is so2 polar
.
Just like with the carbon dioxide example, is so2 polar not only have to take the types of atoms in a molecule of sulfur dioxide into account, is so2 polar, you also have to take the structure of the molecule into account. After this is done, the geometry of the molecule can be determined with the Valence Shell Electron Pair Repulsion Theory VSEPR Theorywhich states that molecules will adopt a geometrical formation that maximizes the distance that the electrons have from one another.
.
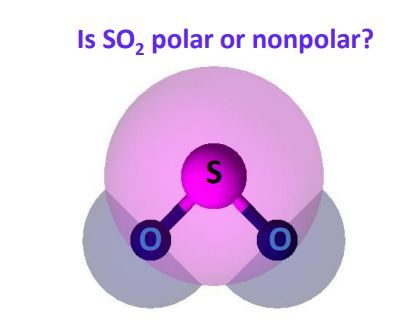
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2. Is SO2 polar or nonpolar? SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms.
Is so2 polar
We learned in Section However, when two nonmetals come together, they will share electrons with each other to form covalent bonds as we learned in Section When describing a covalent bond, the implication was that the two electrons in the bond were shared equally between the two nuclei involved. While this is sometimes true, it seems unlikely that all nonmetal nuclei have identical attractiveness for the electrons. How can we tell if there is a difference and what would a difference do to the bond? The ability of an atom in a molecule to attract electrons is called electronegativity. Electronegativity is a dimensionless number; the greater the electronegativity value, the greater the attraction for shared electrons. Electronegativities are used to determine the polarity of covalent bonds. An interactive version of this table may be found here.
Surface pro won t turn on
Ionic bonds are formed between metals and nonmetals. What about sulfur dioxide , is it polar or nonpolar? Notify me of followup comments via e-mail. This means the oxygen is exerting more pull on the covalent bonds in sulfur dioxide. For instance, in carbon dioxide, the carbon-oxygen bonds are polarized toward the oxygen, which is more electronegative, and since both bonds have the same magnitude their sum is zero and the molecule is classified as nonpolar. Just like with the carbon dioxide example, you not only have to take the types of atoms in a molecule of sulfur dioxide into account, you also have to take the structure of the molecule into account. What influences how electrons are shifted around is the bonds that exist between molecules. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Therefore, one lone pair from each oxygen is used to make an additional bond with the sulfur: The central atom has a steric number of 3 — two atoms and one lone pair. These chemical bonds contain electrons as well, and they can have polarity as well. A difference of 1. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.
The dipole moment is a result of the uneven distribution of negative and positive charges. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. When trying to determine the polarity of a molecule, you can use a three-step process to analyze it. One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Either way, there will be one part of the bond that has a slightly more positive charge and one part of the bond that has a slightly negative charge. By Daniel Nelson. Most alkaline elements have a similar structure to C2H6, and for this reason, it is typically said that alkaline elements are nonpolar. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Our panel of experts willanswer your queries. What exactly does being a poor molecule mean? So in essence, sulfur dioxide is polar while carbon dioxide is nonpolar because the individual movements of the bonds in carbon dioxide cancel one another out, yet in the case of sulfur dioxide, the angular nature of the molecule means that there is an imbalance between the poles — that it has both a negative and positive side — and therefore the molecule is polar. If the difference in electronegativity is less than 0.

You are certainly right. In it something is and it is excellent thought. It is ready to support you.
This topic is simply matchless
I am sorry, I can help nothing, but it is assured, that to you necessarily will help. Do not despair.