Is turmeric olfactory indicator
Last updated on Feb 23, Get Started. English Hindi. This question was previously asked in.
By now, we know that substances like tomatoes, lemon, pineapples taste sour because they contain acids. In contrast, substances like detergents and soaps feel bitter and slippery because they contain the substance known as base. How do we find it acidic or basic if we cannot taste every substance? Special types of substances are used to determine whether a given substance is acid or base. These substances are known as indicators. Indicators change colors when they contact an acid or a base. Let us learn more about these indicators, types and how they are used to identify the nature of substances.
Is turmeric olfactory indicator
Try free sample papers for Olympiads. There are some substances which help us to detect whether a substance is acidic or basic in nature. Such substances are known as indicators. There are some naturally occurring indicators which change their original color when they come in contact with acidic, basic and neutral substances. This is the most common natural indicator which is available in almost every home. It contains a substance called curcumin which gives it a beautiful yellow color. A basic substance will change a yellow colored stain on a cloth of the turmeric into brownish red and an acidic substance will reverse this brownish red stain back into its original yellow color. The petals of China rose are used to make a solution with water which is pinks in color and is a good natural indicator. With the acidic solution, it gives dark pink magenta and with a basic solution, it yields green color. With a neutral medium, it remains as it is with no change in color. Litmus is a natural dye which is extracted from lichens.
With a neutral medium, it remains as it is with no change in color.
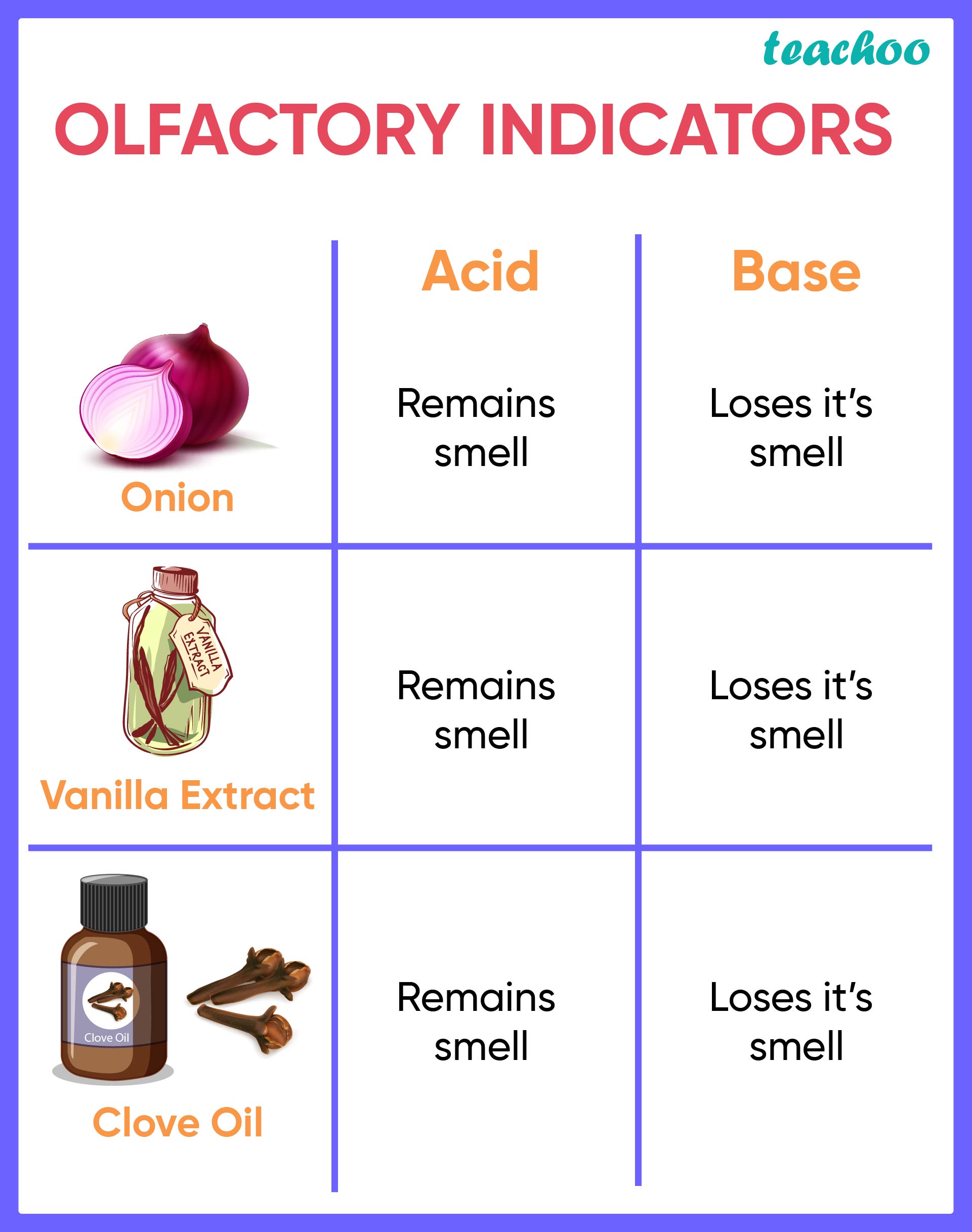
Olfactory Indicators: Substances which change their smell when mixed with acid or base are known as olfactory indicators. For example onion, vanilla, clove, etc. Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid. Turmeric is a common spice in curry.
Chemicals that change colour with pH are frequently used as indictors for acid-base titrations. The aim of this experiment it to determine the concentration of an unknown base using garlic powder as an olfactory indicator. The aim of this experiment it to determine the concentration of an unknown base using garlic powder as an olfactory indicator, and to analyse the accuracy and precision of the results. You have to decide if this experiment is suitable to use with different classes, and look at the need for preliminary training in using techniques involved in tritration. What follows here is an experiment were titration techniques can be practised and polished as it allows for an interesting comparison between indicators and and assessment if accuracy at determining the end-point of this strong acid-strong base titration. If is expected that this class practical will take 60 minute to complete. The chemical quantities suggested in the table will be enough to run 10 titration experiments. Depending on the size of the class and the number of trials you want each group to complete you many need to adjust the quantities to suit. The preparation of garlic infused sodium hydroxide is probably best completed in advance of the lesson. NB: The smell is detectable in basic conditions but at the end point there is a sudden change of intensity whereby the smell seems to get richer and can easily be detected by wafting the odour towards oneself.
Is turmeric olfactory indicator
Chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. This article provides a comprehensive insight into chemical indicators, discussing their definition, types, examples, and importance in various chemical contexts. The most common change is a color shift, but olfactory indicators, which respond by altering scent, are also used. These indicators are essential in gauging the pH levels of solutions, signifying how acidic or basic they are. The pH scale typically ranges from 0 highly acidic to 14 highly basic , with 7 being neutral. Chemical indicators can be broadly categorized into three types: natural indicators, artificial indicators, and olfactory indicators.
Pawn shops victoria tx
Nov 26, Maths English Physics Chemistry more.. The cooking gas is mainly a mixture of the following two gases:. Litmus Litmus is a natural dye which is extracted from lichens. They can exist as atoms or molecules and cannot be divided into smaller pieces. It does not change its smell with acid. Litmus is a natural dye which is extracted from lichens. Important Exam. Please select your grade. Biology Science Earth and space. Suggested Test Series. Need Help? Why is Turmeric yellow?
Olfactory Indicators: Substances which change their smell when mixed with acid or base are known as olfactory indicators. For example onion, vanilla, clove, etc. Onion: Paste or juice of onion loses its smell when added with base.
Olfactory Reference Syndrome The indicator which produces a pink colour in an alkaline solution: a Methyl orange b Turmeric paper c Phenolphthalein d Litmus paper Steam is an example of gas or not? Nimbus Learning. China rose The petals of China rose are used to make a solution with water which is pinks in color and is a good natural indicator. This is because carbon atoms make up the backbone of many important molecules in the human body, including proteins, DNA and RNA, sugars, and fats. However, when it is added to lemon juice or vinegar, the color remains the same. It contains a substance called curcumin which gives it a beautiful yellow color. How to Solve Right Triangles? It is because of turmeric. What is need to be added in turmeric, to make a turmeric solution? Get me the extra edge for Olympiads Exams. Indicators change colors when they contact an acid or a base. The entire field of organic […].

0 thoughts on “Is turmeric olfactory indicator”