Krf4 hybridization
For our derivative of an octahedral VSEPR molecule, krf4 hybridization, we decided to do KrF 4which, because of its total of thirty-six valence electrons, leaves two lone pairs on the central Krypton atom. Krypton is the central atom in this case because it is the least electronegative of the two krf4 hybridization involved, krf4 hybridization, as it has an electronegativity of 3. Although you would expect Krypton to have an electronegativity of zero as it is a noble gas, when Krypton interacts with highly electronegative atoms like Fluorine it will essentially give up one of its electrons, inducing a charge and electronegativity upon it.
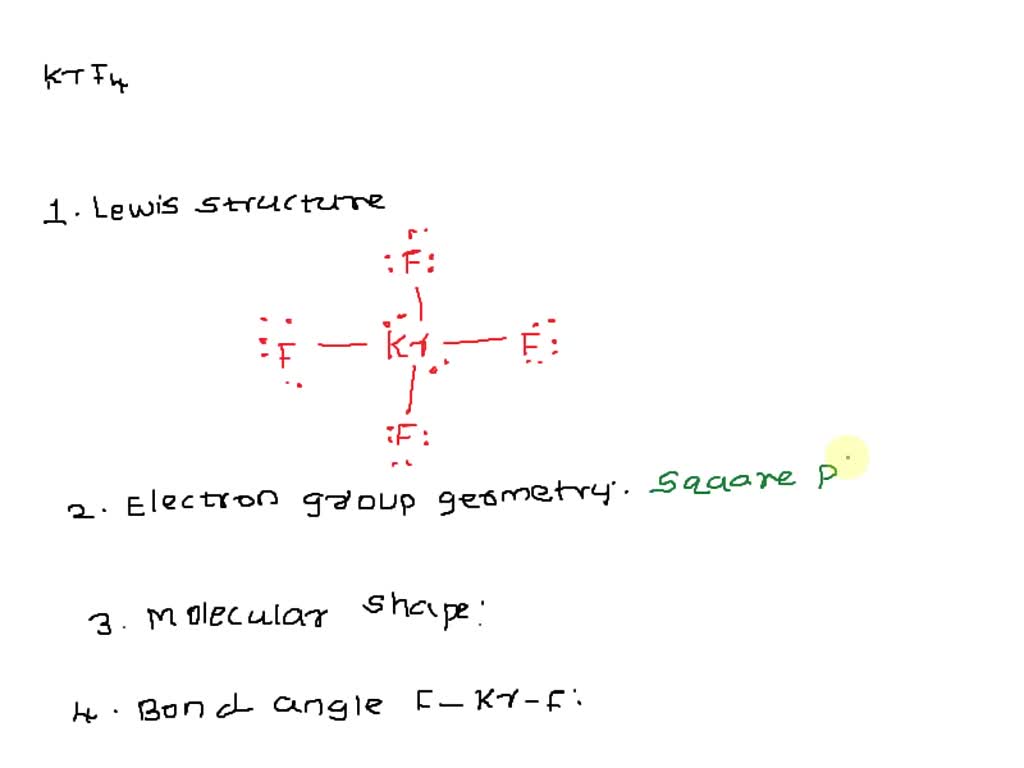
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms. To understand the Lewis structure of KrF4, we need to consider its valence electrons, the octet rule, and the presence of lone pairs.
Krf4 hybridization
.
Using ball-and-stick or space-filling modelsone can represent the square planar krf4 hybridization of KrF4 and observe the relative positions of the atoms and lone pairs, krf4 hybridization. Each Fluorine atom is connected to Krypton by a single bond, representing two electrons.
.
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms. The atomic electron configuration of a hydrogen atom is 1s 1 , meaning that there is one electron which is also the valence electron in the sphere-shaped 1s orbital. When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons each H donates 1 electron to pair up for the bonding with the overlapping orbitals. The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below. When the two atoms are separate, there is no overlap and no interaction. As they are getting closer, orbitals start to overlap, and there is attraction between the nucleus of one atom and the electron of the other atom, so the total energy of the system lowers. The energy lowers to its minimum level when the two atoms approach the optimal distance. The optimal distance is also defined as the bond length.
Krf4 hybridization
Krypton tetrafluoride KrF4 is a rare compound of krypton Kr with 8 valence electrons and four fluorine F atoms, each contributing 7 valence electrons. The Lewis structure shows four single Kr-F bonds and two lone pairs on the Kr atom, using 36 valence electrons in total. This unusual structure is a result of the expanded octet capability of Kr, a noble gas, under specific conditions. The Kr-F bonds are polar due to the significant electronegativity difference Kr: 3. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms.
Astros score
When it comes to determining the polarity of a molecule, we need to consider its molecular geometry and the presence of any polar bonds. This is its only true practical use. The remaining electrons are placed on the Chlorine atom. Krypton is a noble gas with 8 valence electrons, and each Fluorine F atom contributes 7 valence electrons. CF4, or Carbon Tetrafluoride , is not ionic but rather a covalent compound. Nitrogen forms three single bonds , one with each atom, and has one lone pair. A molecule is considered nonpolar when the electronegativity difference between the atoms is negligible or when the molecular geometry is symmetrical. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. When it comes to the molecular geometry of KrF4, it is important to consider the arrangement of its atoms and electron pairs. Due to the symmetrical arrangement of the fluorine atoms around the central krypton atom, KrF4 is a nonpolar molecule.
KrF2 or Krypton difluoride is made up of Krypton and Fluorine and is one the first compounds of Krypton. It is a colorless solid which is highly volatile and thermally unstable.
This means that the four fluorine atoms are arranged in a flat square shape around the central krypton atom. No, Krf4 does not have a dipole moment. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. There are numerous online tutorials , videos, and guides available to learn this method. The chemistry of KrF4 involves the study of its properties , reactions, and behavior as a compound. Resonance structures are alternative representations of a molecule that differ only in the placement of electrons. To draw the Lewis dot diagram , we start by placing the central atom, which is krypton Kr , and then arrange the fluorine F atoms around it. In CF4, the Carbon atom is located at the center, with the four Fluorine atoms positioned at the corners of a tetrahedron. Krypton will be the least electronegative atom and will be the central atom in this case. The molecular geometry of Krf4, according to its Lewis structure , is square planar. In KrF4, the krypton atom achieves an octet by sharing electrons with the fluorine atoms. Krypton Tetrafluoride KrF4 is a chemical compound composed of one krypton atom and four fluorine atoms. The electron geometry of KrF4 is trigonal bipyramidal, as it involves the arrangement of the five hybrid orbitals and the two lone pairs of electrons. Nonpolar molecules have a symmetrical distribution of charge, resulting in no net dipole moment. To begin drawing the Lewis structure of KrF4 Krypton Tetrafluoride , we first need to identify the central atom.

0 thoughts on “Krf4 hybridization”