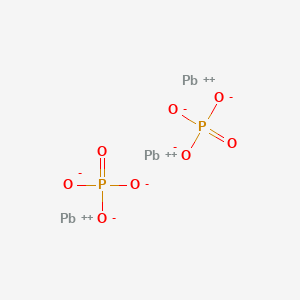
Lead iv phosphate
Wiki User.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Lead iv phosphate
.
Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. Previously Viewed.
.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions Figure 2. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion.
Lead iv phosphate
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions, so that you can write a correct formula.
Aria dazzle
What is the chemical formula of disodium phosphate? Best for: Simple equations with a small number of atoms. Resources Leaderboard All Tags Unanswered. This is the most straightforward method. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Best Answer. Enter a chemical equation to balance:. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Previous Next: balancing chemical equations. Best for: Equations that are more complex and not easily balanced by inspection.
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of silver nitrate was mixed with a yellow-orange solution of potassium dichromate to give a reddish precipitate of silver dichromate:. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble.
Q: What is the formula for Lead IV phosphate? The chemical formula for dihydrogen phosphate ion is H2PO The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character. The chemical formula for rubidium phosphate is Rb3PO4. Tags Elements and Compounds Subjects. It shows the reactants substances that start a reaction and products substances formed by the reaction. What is the chemical formula for rubidium phosphate? Unit converters. Formula: Pb3P4. Still have questions? Phosphorus goes from 0 to -3, gaining 3 electrons oxidation. What is the chemical formula of leadiv oxide? There are 2 H atoms on the left and 2 H atom on the right. Best For: Redox reactions where electron transfer occurs.

In my opinion you commit an error. I suggest it to discuss. Write to me in PM, we will talk.
I can not with you will disagree.