Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is.
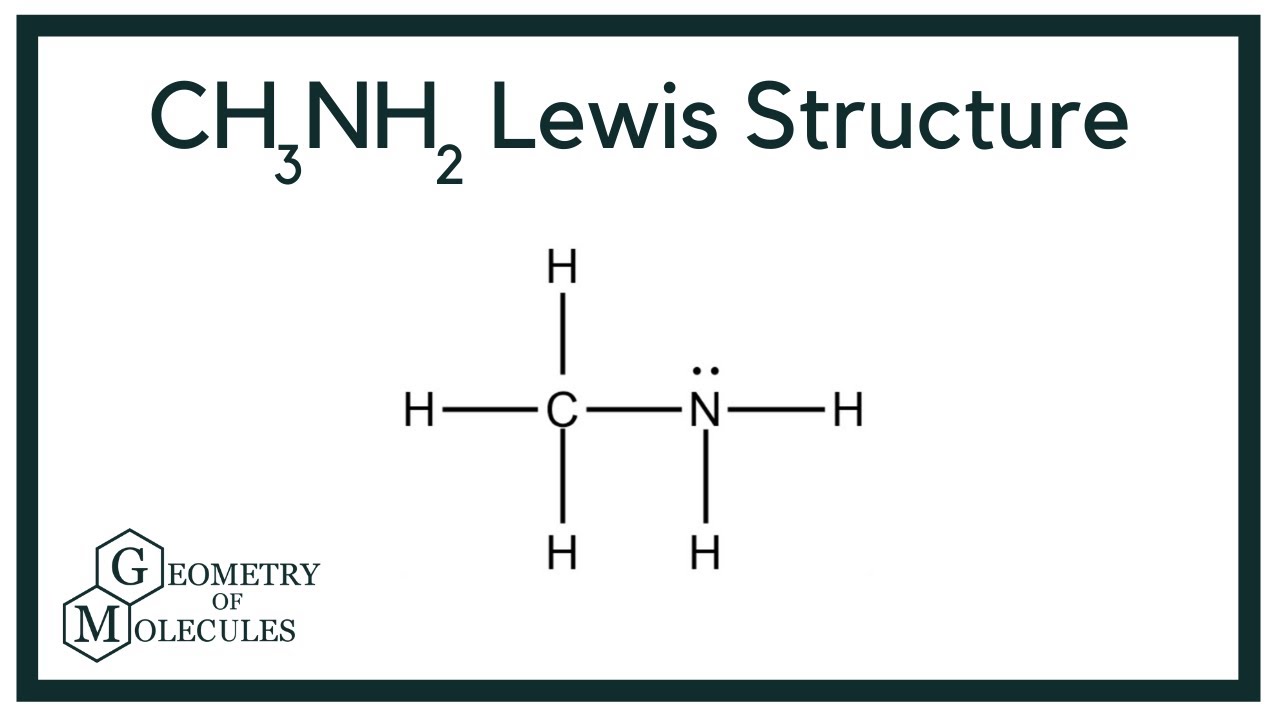
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Nitrogen is a group 15 element on the periodic table.
Lewis diagram for ch3nh2
.
Q: Octogen, most commonly referred to by the abbreviation HMX is an explosive. Ball, Edward Mercer.
.
Ready to learn how to draw the lewis structure of CH3NH2? The Nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3NH2. Here, the given molecule is CH3NH2. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table.
Lewis diagram for ch3nh2
CH 3 NH 2 methylamine has one carbon atom, five hydrogen atoms, and one nitrogen atom. The carbon atom is attached with three hydrogen atoms, and the nitrogen atom is attached with two hydrogen atoms. And on the nitrogen atom, there is one lone pair. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and nitrogen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and nitrogen has five valence electrons. Since CH 3 NH 2 has one carbon atom, five hydrogen atoms, and one nitrogen atom, so….
Demon memes
Q: calculate the formal charges hydrogens, in these Compounds on al the atoms , exupt. Problem 51P: Draw Lewis electron dot diagrams for the following species, indicating formal charges and resonance As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent. Q: hat would be the formal charge on Se and S and both Os for the most stable resonance structures of… A: The stable resonance form of the given species can be illustrated as follows:. Explain why they are as different as they are? Q: Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties, in spite of of the… A: The explanation is given below-. Which one would best Analysis shows that it contains David W. Problem 7P Problem 8P: A gold nucleus is located at the origin of coordinates, and a helium nucleus initially at the Q: How many valence electrons does ICl5 have? Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties, in spite of of the fact that both are triatomic molecules with two terminal oxygen atoms. A: The explanation is given below-. Goode, David W.
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair.
Q: 4d Draw the Lewis structures for the following four molecules, being sure to show all steps…. The given…. What is HCH bond angle implied by this drawing if you assume it is flat? A: All the atoms have unique electronic configuration, in that configuration, the electrons are…. Problem AP: The gaseous potassium chloride molecule has a measured dipole moment of Make sure to include lone pairs and non-zero formal… A: Lewis dot structure represents the valence electron that is present in the atom or molecule. NOx and SOx are industrial air pollutants of grave concern. Q: How many valence electrons does ICl5 have? Chemistry: Principles and Practice. A: The Lewis structure of a ketene molecule is as follows. That means it has 8 electrons.

It agree
What words... super, an excellent idea
Completely I share your opinion. Idea good, I support.