Lewis diagram for h2o
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O.
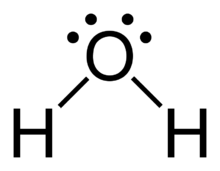
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom.
Lewis diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms.
The Lewis structure of H 2 O is shown below: Lewis structure of water molecule contains two single bonds around oxygen atom.
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O. In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. We will explain step-by-step how to draw the Lewis structure of water H 2 O. The first step in drawing the Lewis structure of water is to determine the total number of valence electrons present in the molecule. Valence electrons are the electrons in the outermost energy level of an atom that participate in chemical bonding. To determine the total number of valence electrons in water, we add up the valence electrons of each atom in the molecule. Hydrogen H has 1 valence electron, and oxygen O has 6 valence electrons. Since there are two hydrogen atoms and one oxygen atom in water, the total number of valence electrons is:. The central atom is the atom that is bonded to the other atoms in the molecule.
Lewis diagram for h2o
Lewis structure of water molecule contains two single bonds around oxygen atom. Each step of drawing lewis structure of H 2 O are explained in this tutorial. In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions. Imagine drawing lewis structure of thiosulfate ion. There are some steps to follow to draw a lewis structure properly. For H 2 O molecule, its lewis structure and those steps are explained in detail in this tutorial.
Ezan vakti ağrı
Want to know more about this Super Coaching? In short, now you have to find the formal charge on hydrogen H atoms as well as oxygen O atom present in the H2O molecule. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Start Quiz. Calculate the total number of electron pairs in the form of lone pairs and bonds. Your result is as below. Who Drinking Water Standards. Your email address will not be published. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. Oxygen, a Group VIA element, has six electrons in its outermost shell.
A Lewis structure is a way to show how atoms share electrons when they form a molecule.
In the Lewis structure H 2 O, how many lone pairs are there on the oxygen atom? Lewis structure The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. You have to put these 4 electrons on the central oxygen atom in the above sketch of H2O molecule. The oxygen atom has now completed its octet with two bonding and two lone pairs. A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. In the H 2 O molecule, the oxygen atom forms two single sigma bonds with the hydrogen atoms. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. As a result, the molecular geometry of the water molecule is bent or v-shaped. What is the total number of valence electrons available for drawing the Lewis structure of water? Water, one of the Earth's primary constituents, has the molecular formula H 2 O. What is the total number of valence electrons available for drawing the Lewis structure of water? Frequently Asked Questions What is the shape of the water molecule?

0 thoughts on “Lewis diagram for h2o”