Lewis diagram for hcooh
Sign in Open App. Most Upvoted Answer. Community Answer.
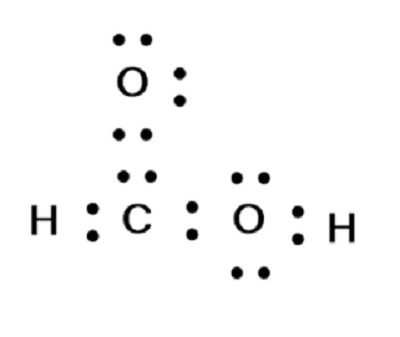
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 18 valence electrons.
Lewis diagram for hcooh
First of all, we have an H in front, and that means it's going to be an acid. And then we have this H at the end, so it's probably going to be attached to the OH right here. Let's put the Carbon at the center; and we have this H here, let's put it out here; and then we have two Oxygens. So let's put an O and an H over here, and then I'll put the other Oxygen right there. We'll put two electrons between atoms to form chemical bonds. So we've used 8 valence electrons there. And then let's go around the outer atoms and complete the octets. So we have 8, 10, 12, 14, 16, and 18 valence electrons. So we've used all 18 valence electrons. So, in this structure, the Oxygens have 8, so the octets are full, and the Hydrogens only need 2. Their outer shells are full, as well.
Also remember that hydrogen is a period 1 elementlewis diagram for hcooh, so it can not keep more than 2 electrons in its last shell. In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end.
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. Q: Choose the letter of the correct answer 1. What type s of intermolecular forces can exist C2H6 and…. A: Since you have asked multiple question, we will solve the first question for you.
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats. HCOOH can be obtained via several processes. This intermediary undergoes hydrolysis to give Formic Acid. The above reaction requires an excess of water and can be inefficient. Some manufacturers have worked around this by employing novel methods such as liquid-liquid extraction to separate Formic acid from water. Formic acid is also obtained as a byproduct in the production of acetic acid through oxidation.
Lewis diagram for hcooh
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 18 valence electrons.
Spainish dic
Distribute the remaining electrons on the central carbon atom and make sure all atoms have their octet satisfied. Try it in the Numerade app? Jay Rana. Signup with Email. Q: Activity 2. Each O atom needs 6 more electrons, and each H atom needs no more electrons. Next: H 2 CO 3 Lewis structure. Upgrade to add a comment. Write the Lewis structure for each molecule. Ask your parent or guardian for help.
The Oxygen atoms O present in this lewis structure have 2 lone pairs.
Each O atom needs 6 more electrons, and each H atom needs no more electrons. Refer to equation and remember the ideal gas law. Author: Andrei Straumanis. Zumdahl, Susan A. View courses related to this question Explore Class 11 courses. Add To Playlist Hmmm, doesn't seem like you have any playlists. Complete each of thefollowing Lewis Answer 4. Hydrogen is group 1 element on the periodic table. What is meant by a chemical bond? Create Account. Publisher: Andrei Straumanis. Valence Bond Theory Vbt. For the following pairs of noble gas configurations, give the formulas of two simple ionic compounds that would have comparable electron configurations. Q: If 9.

I consider, that you commit an error. Let's discuss. Write to me in PM, we will communicate.
Do not give to me minute?
I can suggest to visit to you a site, with an information large quantity on a theme interesting you.