Lewis dot for ch3oh
Q: Draw the Lewis structure for a chlorate ion ClO A: Lewis structure is the simplified way to represent how electrons are arranged around an individual…. A: Lewis Dot Structure are those structures in which the electrons are represented by the dots and the…. Draw a possible Lewis structure for one of…, lewis dot for ch3oh.
We start with the Lewis Stru Compared with the monster seas of the Pacific, Arctic waters are a picture of calm—whipping up, at their most violent, into lake-like chop. Or, at least, they were. New research sh It provides valuable insights into the …Oct 22,
Lewis dot for ch3oh
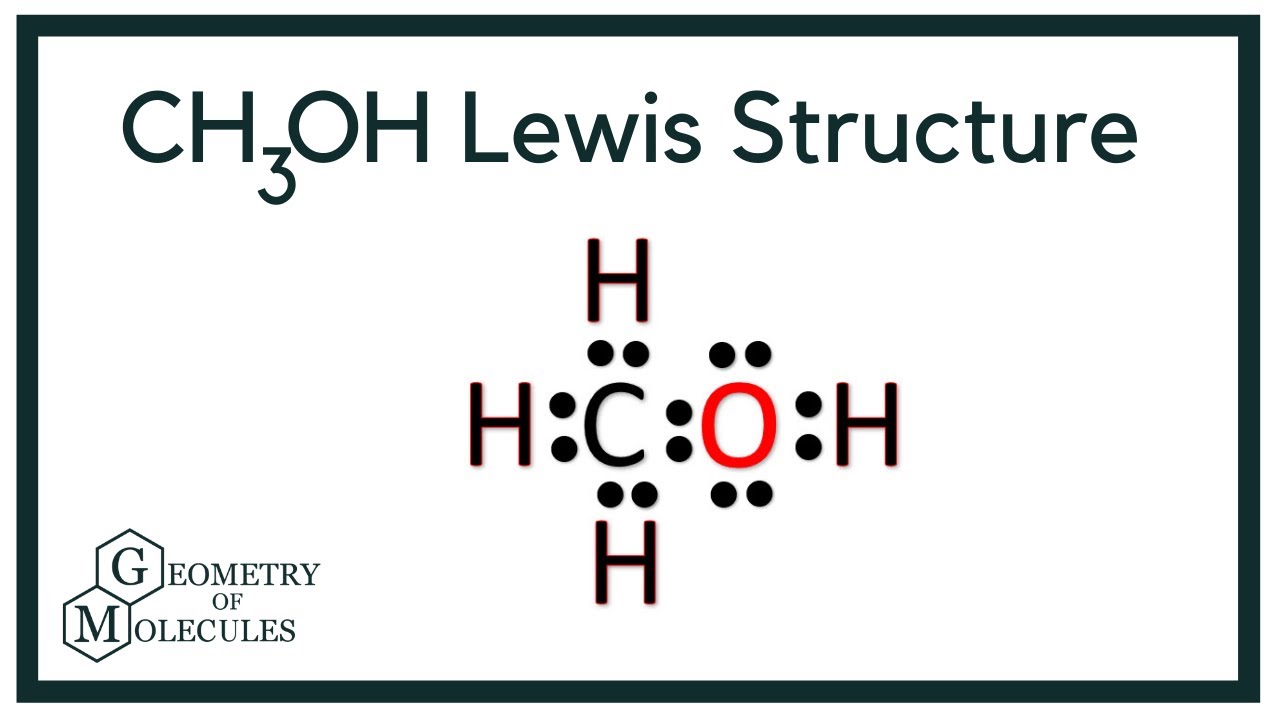
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen. Hydrogen is a group IA element and has only one electron in its valence shell. Oxygen belongs to the group VIA and has six electrons in its last shell valence shell. Carbon atom has 4 electrons in its last shell because it is a IVA group element. Now we know how many electrons are includes in valence shells of atoms. To find out total valence electrons given by a particular element in a molecule or ion, you should multiply number of electrons of the valance shell by the number of atoms of that element in respective molecule. Total electron pairs are determined by dividing the number total valence electrons by two. To be the center atom, ability of having greater valance and being most electropositive element in the molecule are leading facts. However, CH 3 OH is a simple molecule.
Give the Lewis dot structure of BF3. Yes, the structure is not symmetrical meaning that it is polar.
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. Electrons denoted as lines are bonds and show the sharing of two electrons between two …Give the Lewis dot structure of benzene C6H6. Give the Lewis dot structure of CH4. Give the Lewis dot structure of NH3.
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen. Hydrogen is a group IA element and has only one electron in its valence shell. Oxygen belongs to the group VIA and has six electrons in its last shell valence shell.
Lewis dot for ch3oh
Ready to learn how to draw the lewis structure of CH3OH? The oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Auto repair shop for sale
Tools Tools. What is the Lewis Structure? Figure 3. The calculation should take about twelve seconds to complete. Index for ALL chemical bonding and structure notes. A: Lewis structures are in fact electron dot diagrams, that represent the bonding between atoms. It is commonly used as a polar solvent and in Process and plant safety. Q: A compound has the formula C8H8 and does not contain any double or triple bonds. To determine whether CH3OH methanol is polar or nonpolar, we need to examine its molecular structure and the distribution of its electrons. A single line is used to represent one pair of shared Show each step of your calculations. Use carbon tetrachloride, CCl4, as an example. The four C-Cl bonds are polar, but they are arranged in a tetrahedral geometry, which results in a non-polar molecule.
CH 3 OH methanol has one carbon atom, four hydrogen atoms, and one oxygen atom. And on the oxygen atom, there are two lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group
This chart asks students to record the data for each of the hybrid orbitals. Jerry; Tang, Yui S. Q: Draw the Lewis structure for PCl3. GABA A receptor positive modulators. Recommended textbooks for you. Oxygen has more electron density due to its two lone pairs of electrons. So the correct option is B. Accounts are free of charge, and instructions for obtaining an account are available at this site. Skeletal formula of acetone. The teacher should use this activity to assess student's ability to construct Lewis dot structures, and to assist them in visualizing what these structures look like chemically. Finally, check to see if the total number of valence electrons are present in the Lewis structure. Author: John W. In which solvent are each of the following more likely to be soluble? A: Given formula of the compound is C8H8. Lewis structures — also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures LEDs — are diagrams that show the …A step-by-step explanation of how to draw the CO2 Lewis Dot Structure Carbon dioxide.

Now that's something like it!
Has casually come on a forum and has seen this theme. I can help you council. Together we can come to a right answer.
What do you advise to me?