Lewis dot of h2s
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H.
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons of each atom:. Identify the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside.
Lewis dot of h2s
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding. However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour. The acidic portion of ethanoic acid is a monocarboxylic acid containing two carbons. Besides regulating the acidity of food, it is also used as an antimicrobial food preservative and a Daphnia magna metabolite. It is an acetate conjugate acid. The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms. The sulphur atoms also have two lone pairs. The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms.
But as per the rule we have to keep hydrogen outside. The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms.
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom. The central atom must have a high valence or minimal electronegativity.
Transcript: All right, this is Dr. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing.
Lewis dot of h2s
H2S or hydrogen sulfide gas is colorless in nature. With many other various pet names like sour gas, sewer gas, etc this gas is poisonous and corrosive as well. I am sure you are not expecting a good odor from this gas! Well yes, you are right, hydrogen sulfide gas smells like rotten eggs!! The molar mass of H2S is H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond.
Which two teams lost the most games in ipl 2022
This indicates that the above lewis structure of H2S is stable and there is no further change in the above structure of H2S. Hydrogen is group 1 element on the periodic table. Each hydrogen atom has two electrons around it and also reaches a steady state. However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour. Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Begin by adding lone pairs dots around each hydrogen atom, fulfilling their duet rule. Hydrogen Sulfide manufacturers. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms. Now in the H2S molecule, you have to put the electron pairs between the sulfur atom S and hydrogen atoms H. About author. In the Lewis structure of H2S hydrogen sulfide , the sulfur S atom forms an octet with 8 electrons in its valence shell. Trending Questions. Distributing Remaining Valence Electrons. When fluorine atoms combine, each fluorine shares 1 electron, forming a single bond between them.
Hydrogen sulfide H 2 S molecule consists of one sulfur S atom and two hydrogen H atoms. Hydrogen H is located in Group 1, and sulfur S is in Group 16 of the periodic table.
It is an essential component for energy production in cells D-ribose is an aldehyde containing pentose sugar and is present in all living cells. Scroll to Top. These outer hydrogen atoms are forming a duplet and hence they are stable. Hydrogen atoms only require two electrons to achieve a full outer shell, while sulfur needs eight electrons. Yes, H2S is a polar molecule due to the asymmetrical distribution of atoms and lone pairs, resulting in an overall dipole moment. Sum the valence electrons of each atom:. When fluorine atoms combine, each fluorine shares 1 electron, forming a single bond between them. The Lewis structure of H2S is shown below: Steps for drawing the H2S Lewis structure Step 1 Calculate the number of valence electrons for S and H Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Identify the Central Atom.

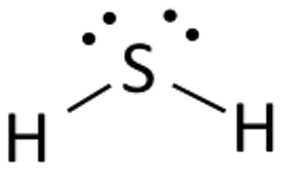
0 thoughts on “Lewis dot of h2s”