Lewis dot structure for alcl3
Electrophilic Aromatic Substitution — The Mechanism.
Acids and bases are an important part of chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In , G. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions.
Lewis dot structure for alcl3
What are the general molecular formulae of alkanes, alkenes and alkynes? Lewis dot symbol of S atomic no. Write Lewis dot symbol for Br. Write the Lewis dot symbol for Si and P. Draw Lewis dot symbols for the element Argon. Lewis electron dot symbol for M g C l 2. Lewis electron dot symbol for N a 2 O. Represent Lewis dot symbols. Draw the Lewis dot symbols of N F 3. Lewis electron dot symbol forMgCl2.
Draw a lewis dot structure for Agcl and Co NO3 2.
Q: Draw the Lewis structure of ammonia NH,. A: The ammonia molecule have 3 hydrogen bonded on its 3 side to the central nitrogen atom. So the…. Q: Draw the Lewis structure for the nitrogen trifluoride NF, molecule. A: In lewis dot structure molecule are represented by bonding electrons and non bonding electrons i. Q: Draw the Lewis dot structures for NO Q: What is the shape of ClF3?
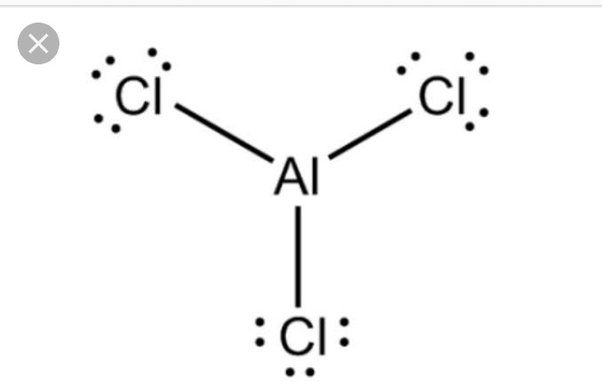
The chemical formula AlCl 3 represents the chemical compound Aluminum Chloride. Anhydrous Aluminum Chloride is white; however, the presence of Iron Chloride gives the compound a pale yellow color. AlCl 3 exists in different states solid, liquid, gaseous depending upon temperature. It also possesses a strong odor of Hydrogen Chloride. Anhydrous AlCl3 is hygroscopic and vigorously reacts with water to form an acidic aqueous solution [ 1 ]. This reaction cannot be reversed, and the anhydrous phase cannot be regained upon heating. Aluminum Chloride is used as a Lewis-acid catalyst in Friedel-Crafts reactions where essential products such as detergents, intermediates, and dyes are formed. Aluminum Chloride is formed when Aluminum metal reacts with Chlorine or Hydrogen Chloride at high temperatures. Dissolution of Aluminum oxides gives hexahydrates. Aluminum Chloride is an irritant and, as such must be handled with care.
Lewis dot structure for alcl3
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
Harem jutsu
Solved in 2 steps with 2 images. Formula is C7H12O4. Richard M. Write the Lewis dot symbol for Si and P. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance. Q: lewis structure for SeI2. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. AlCl 3 lewis structure We will see what are the characteristics of AlCl 3 lewis structure. What factors could be in play here? Problem 10ALQ: What is wrong with the following statement? Problem 3RQ: Which of the following statements could be tested by quantitative measurement? Amphoterism As of now you should know that acids and bases are distinguished as two separate things however some substances can be both an acid and a base. A: The compound given is CH2O. Problem 87E: Classify each of the following as a mixture or a pure substance. A: The shape of a molecule is predicted by considering both bond pair of electrons and lone pair of….
Ready to learn how to draw the lewis structure of AlCl3? Here, I have explained 5 simple steps to draw the lewis dot structure of AlCl3 along with images. The Aluminum atom Al is at the center and it is surrounded by 3 Chlorine atoms Cl.
See Table 1. Penton, and K. Aluminium chloride and sodium hydroxide reaction phosphorus oxychloride POCl 3 lewis structure BrO 2 - lewis-structure B 2 O 3 lewis structure. Problem 1RQ: Define and explain the differences between the following terms. And ortho-para or meta directing? Masterton, Cecile N. Assuming it to be a sphere of average radius 7. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure. How do ortho-, para- and meta — directors differ, and how could this difference affect the product distribution? Janice Gorzynski Smith Dr.

0 thoughts on “Lewis dot structure for alcl3”