Lewis dot structure for hcn
There is a formal negative charge associated with this anion. Where does it reside? The nitrogen nucleus has 3 electrons from the triple lewis dot structure for hcn, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral.
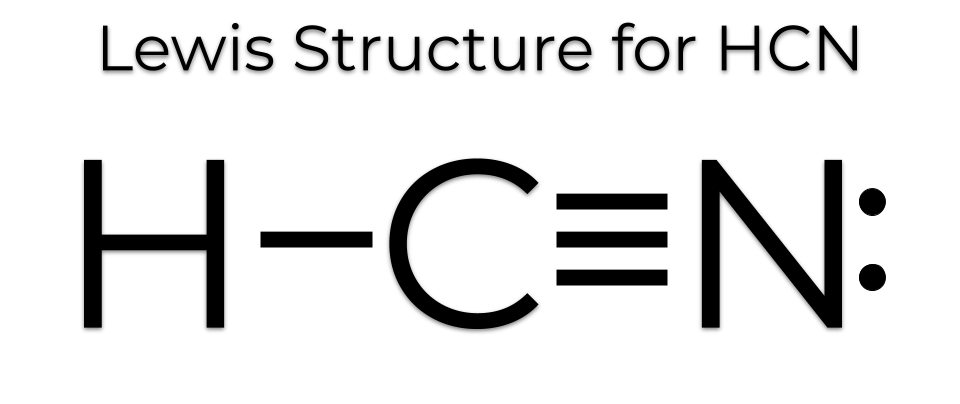
H-CN: Hydrogen forms a single bond with Carbon and carbon a triple bond with nitrogen, with 1 lone pair on the other side of N. There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, : , each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, , representing three pairs of shared electrons, and a lone pair of electrons on the nitrogen atom, represented by a pair of dots, :. I need help with lewis diagram of HCN - which one do I put in the middle? Jared Vincent L. Feb 23, The least electronegative atom is usually the one in the middle, in this case C. You're welcome.
Lewis dot structure for hcn
We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures. We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go around the Nitrogen, six, eight, and ten. So when we look at the Lewis structure, Nitrogen had eight valence electrons, but the Carbon only has four. So we're going to need to move some valence electrons from the center to form a double bond with Carbon. Let's try and do that. Now you can see that Nitrogen has eight valence electrons and Carbon has six. So let's move another pair to the center. So we're still using ten valence electrons for the HCN Lewis structure, but Nitrogen has an octet with eight valence electrons, Carbon has eight valence electrons, Hydrogen has only two but that's all it needs for a full outer shell. Be sure that you don't use more than the ten valence electrons available. See the Big List of Lewis Structures. Opens New Window. So that's the Lewis structure for HCN.
The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell, lewis dot structure for hcn. In ionic solids, the oppositely charged ions are closely packed in spa How do you find density in the ideal gas law?
Explain what is wrong with each one and give a correct structure for the molecule. Relative positions of atoms are shown correctly. Interpretation : The Lewis structures of the molecules should be corrected with appropriate explanation. Concept Introduction: Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds. It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. Keep reading this post to find out its shape, polarity, and more. First, let us look at its Lewis dot structure and the valence electrons that participate in forming bonds.
Lewis dot structure for hcn
Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. HCN has a very strong and pungent smell which is not favorable for humans. The smell can be categorized as being that of bitter almonds.
Is riviera nayarit safe
Let's try and do that. Hydrogen, oxygen, carbon and fluorine contribute 1, 6, 4 and 7 electrons respectively making the total number of valence electrons Bond enthalpies of The carbon atom has or shares 3 electrons from the triple bond, and a lone pair of electrons, which it owns. There is a formal negative charge associated with this anion. Author: Steven S. Write the Lewis dot structure of C O molecule. To what elements does Chapter 9, Problem 9. Can Lewis structures predict the shape of a molecule? Polarity Of Water. Why is lithium iodide more covalent than lithium fluoride? Since there are 3 fluorine atoms the total valence electron of the molecule becomes Impact of this question views around the world.
.
Interpretation Introduction. Carbon is placed as the central atoms since its electronegativity is less than nitrogen. In this structure, there is a lone pair of electron on boron atom whereas in actual structure that lone pair is no needed to fill the octet. Are non-valence electrons represented in a Lewis dot diagram? Consider the following incomplete Lewis structure for an organic compound called histidine an amino acid , which is one of the building blocks of proteins found in our bodies: Draw a complete Lewis structure for histidine in which all atoms have a formal charge of zero. Write two resonance structure of N2 O that satisfy the octet rule. In the given structure, there is a double bond between oxygen and fluorine which is not needed. The total number of valence electrons found to be 10, where 1 electron, 5 electrons were contributed by each H and C atoms respectively. Distributing a lone pair on oxygen is enough to fill its octet. Draw the Lewis structure of C l O 4 per chlorate ion. In the given structure, the carbon contains lone pair of electrons and the bond between carbon and nitrogen is double bond. Two Theories of Bonding. The electronegativity of oxygen atom is less than fluorine and the molecule is with hydrogen and fluorine atoms at the terminal position of oxygen.

You commit an error. I can prove it. Write to me in PM, we will discuss.
It was and with me. Let's discuss this question. Here or in PM.
Not your business!