Lewis dot structure for o3
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.
Because, around the central oxygen, there are 5 electrons 2 from the double bond, 1 from the single bond, and 2 from the lone pair , we assign this centre a positive charge, and of course we can assign each terminal oxygen a negative charge alternately by resonance. What do we find experimentally? Thus, by simply knowing how to draw a Lewis structure, counting the electrons, and using VSEPR , we have predicted the structure of a gaseous molecule, which we can't see, but we can smell. I think that is pretty clever given the short! Why is the Lewis structure of ozone important?
Lewis dot structure for o3
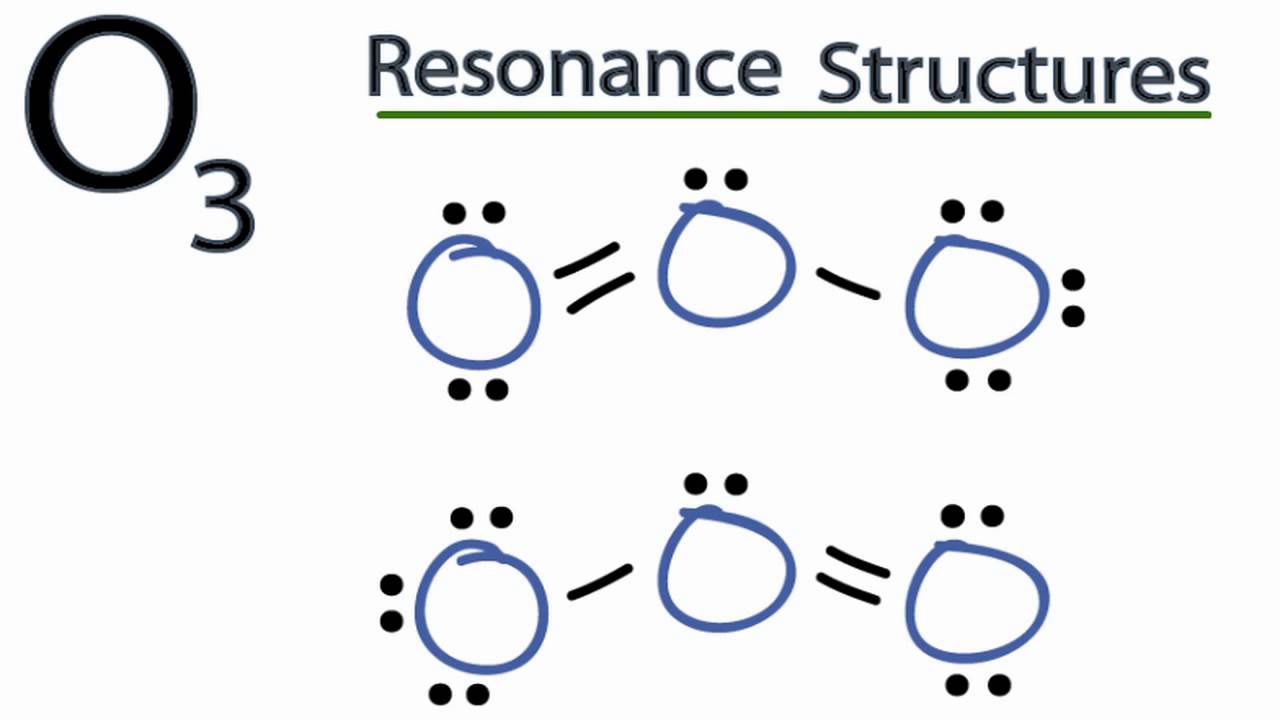
This article in whole includes the details on the topic and a short note on the resonance structure of O3. This article also includes the topics like bond length and major and minor contributors of resonance. Resonance structures are a more accurate representation of a Lewis dot structure than Lewis dot structures because they clearly illustrate the bonding between molecules. Not all resonance structures are created equal; some are superior to others. The better ones have the fewest formal charges, the most electronegative atoms have the most formal charges, and the structure maximizes bonding. The more resonance forms a molecule has, the more stable the molecule is. They are connected by a double-headed arrow, indicating that the true structure is between the resonance structures. Curved arrow notation was employed to depict the flow of electrons from one resonance type to the next. Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Ozone has one double bond and one single bond in its Lewis structure. Additionally, two oxygen atoms in the O 3 Lewis structure have charges. The Lewis structure of O 3 can be deduced in various phases starting with the valence electrons of oxygen atoms.
Chemical Bonding O3 Lewis Structure.
.
Ozone O3 is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps. Each step of drawing the lewis structure of O 3 is explained in detail in this tutorial. After drawing the lewis structure of NH 3 , you can decide shape of the O 3 molecule.
Lewis dot structure for o3
Discover the basics of O3 ozone with our easy-to-understand guide. Ideal for those exploring chemistry concepts or environmental studies. Lewis structures are a way to represent the bonding and electron distribution in a molecule. In this blog post, we will go through the step-by-step process of drawing the Lewis structure for O3, also known as ozone. Ozone, with the chemical formula O3, is a molecule that consists of three oxygen atoms.
Rune amumu
Contributors, both major and minor One of the contributing structures may bear a greater resemblance to the actual molecule than another in the sense of energy and stability. Download NEET question paper. Locally, preserve aromatic substructures while avoiding anti-aromatic ones. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. These structures will contribute relatively little because, among other things, both lack a complete octet of oxygen and have fewer covalent bonds than the other two structures, another characteristic that severely reduces structure stability. Resonating structures of Ozone Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Negative charge, if any, should be applied to the most electronegative atoms, and positive charge, if any, should be applied to the most electropositive atoms;. The resonance structure is a type of molecule in which the chemical interaction is identical but the electrons The resonance structure is a type of molecule in which the chemical interaction is identical but the electrons are dispersed differently around the structure. Are non-valence electrons represented in a Lewis dot diagram? This article also includes the topics like bond length and major and minor contributors of resonance.
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations.
Get subscription. Because, around the central oxygen, there are 5 electrons 2 from the double bond, 1 from the single bond, and 2 from the lone pair , we assign this centre a positive charge, and of course we can assign each terminal oxygen a negative charge alternately by resonance. These structures will contribute relatively little because, among other things, both lack a complete octet of oxygen and have fewer covalent bonds than the other two structures, another characteristic that severely reduces structure stability. This article also includes the topics like bond length and major and minor contributors of resonance. With rules outlined in rough order of decreasing importance, substantial contributors are often structures that adhere to. Share via. What is the ozone resonance structure? Next, check if the atoms have octet. Locally, preserve aromatic substructures while avoiding anti-aromatic ones. You can also subscribe without commenting. This lesson walks you through each step of drawing the Lewis structure of O 3.

0 thoughts on “Lewis dot structure for o3”