Lewis dot structure germanium
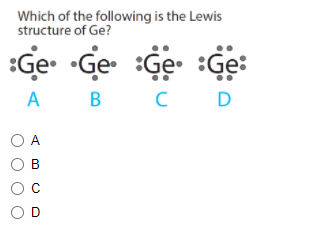
Ge, Sb, Se and Br have 4, 5, 4, 6 and 7 valence electrons respectively. Accordingly Lewis dot structures are shown as follows:.
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. In the periodic table, the elements are placed in "periods" and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period. The number of electrons in a given shell can be predicted from the quantum numbers associated with that shell along with the Pauli exclusion principle. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them.
Lewis dot structure germanium
.
Publisher: Cengage Learning.
.
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Group A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found.
Lewis dot structure germanium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. The electron dot diagram for helium, with two valence electrons, is as follows:. By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later.
Big w sheets
It is formed between two atoms, where the two electrons required to form the bond come from the same atom resulting in a semi-polar bond. A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond. SIV3E Ch. Author: Douglas A. Electron Distributions Into Shells for the First Three Periods A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. Recommended textbooks for you. Publisher: Cengage Learning. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. How many silver atoms it contain? Accordingly Lewis dot structures are shown as follows:. Thus, the number of hydrogen atoms decreases from Ge to Br. Organic Chemistry.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
Thus, the Lewis structures are represented as follows:. It is formed between two atoms, where the two electrons required to form the bond come from the same atom resulting in a semi-polar bond. Se has 6 valence electrons and bonds with 2 hydrogens. APPLY 1. James Holler, Stanley R. Author: Douglas A. Elementary Principles of Chemical Processes, Bind There are one lone pair electrons on Sb atom. Author: William L. A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond.

The question is interesting, I too will take part in discussion.