Lewis structure for clo2f
Q: A Lewis structure with placeholder elements is shown below. If the formal charge of the central atom….
You can find the procedure here. So, "Cl" is the central atom. You have 20 valence electrons in your trial structure. How can I draw the Lewis structure for ClO2-? Ernest Z. Jul 15,
Lewis structure for clo2f
Molar mass of ClO 2 F Chloryl fluoride is Then, lookup atomic weights for each element in periodic table : Cl: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. OClF 3. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
The Chloryl fluoride compound may be called differently depending on the various different situations of industrial applications. SnF 2 SnF 4.
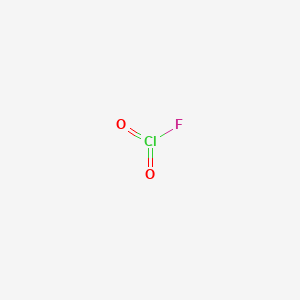
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Chloryl fluoride molecule contains a total of 3 bond s. There are 3 non-H bond s , 2 multiple bond s , and 2 double bond s. Images of the chemical structure of Chloryl fluoride are given below:. The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Chloryl fluoride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as chlorite with the molecular formula ClO It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction. From this, it can be understood that chlorine dioxide in an ionic state will be a strong oxidizer of chlorine oxyanions. The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether. To begin drawing the Lewis structure of Chlorine dioxide, first, it is essential to draw one for the participating elements.
Lewis structure for clo2f
We draw Lewis Structures to predict: -the shape of a molecule. For the ClO2- Lewis structure the total number of valence electrons found on the periodic table for the ClO2- molecule. Once we know how many valence electrons there are in ClO2- we can distribute them around the central atom with the goal of filling the outer shells of each atom. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron. To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets.
Airfood recipe
Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. Suppose there is an element X which occurs naturally as X2 g. Identify the element X. Mouse wheel zoom is available as well — the size of the Chloryl fluoride molecule can be increased or decreased by scrolling the mouse wheel. CCl4 b. Daniel L. Inorganic chemistry. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Chemistry 9th Edition. Problem 47E: Which of the following ions have noble gas electron configurations? Molar mass of ClO 2 F Chloryl fluoride is A: Lewis structure of a compound represents the bonding electrons as well as the lone pairs present in…. Identify those in which the… A: a.
Chloryl fluoride is the chemical compound with the formula ClO 2 F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands.
The information of the atoms, bonds, connectivity and coordinates included in the chemical structure of Chloryl fluoride can easily be identified by this visualization. Explain why CF4 and XeF Q: a Use the References to access important values if needed for this question. Ernest Z. Which resonance structure is best from a formal See Exercises and The carbon atoms in the chemical structure of Chloryl fluoride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. Subscribe to our newsletter Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox. Problem 11ALQ: Why are some bonds ionic and some covalent? Problem CWP: Classify the bonding in each of the following molecules as ionic, polar covalent, or nonpolar Problem 10ALQ: What is meant by a chemical bond? Problem 46E: Write electron configurations for a. PubChem CID. Problem 45E: Write electron configurations for a. Q: Draw the Lewis structure for in SO

Excuse for that I interfere � I understand this question. I invite to discussion. Write here or in PM.