Lewis structure generator
The Lewis Structure Builder is a web application designed to help guide learners in discovering the rules of assembling Lewis Structures for molecules. This app is designed to help illustrate the basics of building Lewis Structures. Visit the menu at any time to change which molecule you're lewis structure generator on, or to get help.
Practise drawing the Lewis structure of molecules using the exercises below. Select answers to see the correct drawings. RMIT Australia. Getting started at uni What will I do? What will I need?
Lewis structure generator
The Lewis Structure calculator is used to draw the Lewis dot structures of various molecules. It takes the molecule as input and outputs its Lewis dot structure. The Lewis theory is a theory proposed by Lewis for covalent bonding and drawing structures of the various compounds. Many other theories were proposed after this theory for covalent bonding. It shows the valence electrons of each atom in a molecule according to the octet rule in the Lewis Structure model. The calculator shows the elaborate structure of the molecule entered in its input window. The Lewis Structure calculator is an online tool that is used to configure the atoms in a molecule for covalent bonding. Covalent bonding is an important concept in the field of chemistry. It is studied by many chemists in history and many theories have been proposed. The first theory proposed for covalent bonding was the Lewis theory. A covalent bond is defined as the sharing of electrons between atoms of a molecule. A single covalent bond results in the sharing of a single electron from both the atoms and double covalent bond results in the sharing of two electrons from both the atoms in a molecule and so on. To understand the Lewis Structure Calculator, the user needs to understand the Lewis theory for covalent bonding. The Lewis structure is based on two principles. The first principle of the Lewis model states that the structure is drawn only considering the electrons in the outer-most bond.
The calculator shows the elaborate structure of the molecule entered in its input window. What Is a Lewis Structure Calculator? Drag atoms into positions next to other atoms where they can be bonded to each other, lewis structure generator.
.
The Lewis structure generator sounded challenging to understand, but it is not so complicated if you get the depth of this structure. Without knowing it, you will not get an exact idea on how to use this link. Let's see, what it is all about, and why should you learn about this? As per the chemistry concept, it is a graphical or pictorial representation of the electron distribution around the atoms. The main reason to learn about this is to project the number and type of bonds that may be generated around a bit in any chemical reaction. It also helps to create a projection regarding the molecule geometry. As it is a chemical concept, it is evident that many chemistry students get confused about these models while generating these structures online.
Lewis structure generator
This simulation provides practice in building Lewis Dot Structures from atoms and ionic compounds from the component ions. Show or hide the tutorial. Show or hide the direction pad to move atoms around the work area. Undo the last bond. Clear the workspace. Reset the current molecule. Check your answer. Select an atom with an unpaired electron to activate it for bonding. Select the same atom again to deactivate it.
Homes for sale in allenhurst nj
The nitrogen octet now consists of six electrons, its octet is not complete. It demonstrated the basic structure which many chemists studied further to invent new theories related to covalent bonding. Learning activities. New to university? Help Video Introduction. About the institution. The input interpretation shows the name of the molecule which the user entered in the input tab. The calculator shows the elaborate structure of the molecule entered in its input window. When ready, check your work to complete the molecule or get feedback. They do not deal with the sharing of electrons. The molecule contains the atoms of the same element whereas a compound contains atoms of various elements. Referencing What is referencing? If the molecule contains a positive charge , it will always be on the central atom.
Atoms are represented by their chemical symbols, and valence electrons are shown as dots around the symbols or as lines between symbols representing shared electron pairs. Lewis diagrams help illustrate bonding and electron distribution in molecules. The Lewis Diagrams provide a basic visual representation of how the atoms are connected and how valence electrons are distributed.
Referencing What is referencing? The structure of the molecule is formed by first selecting the central atom. Hydrogen has the exception of a maximum of two electrons in the valence shell. Getting started at uni What will I do? The Output window of the Lewis Structure Calculator shows the following tabs in the result window:. This app is designed to help illustrate the basics of building Lewis Structures. The Lewis theory is a theory proposed by Lewis for covalent bonding and drawing structures of the various compounds. Four hydrogen atoms surround the nitrogen atom by four single covalent bonds. About the institution What will I need? Through the single covalent bonds , nitrogen has complete eight electrons in the valence shell and hydrogen has complete two outer-most electrons as shown in figure 1. Studying efficiently Time management Mind mapping Note-taking Reading skills Argument analysis Preparing for assessment Critical thinking and argument analysis Online learning skills Starting my first assignment Researching your assignment. The Lewis Structure Builder is a web application designed to help guide learners in discovering the rules of assembling Lewis Structures for molecules.

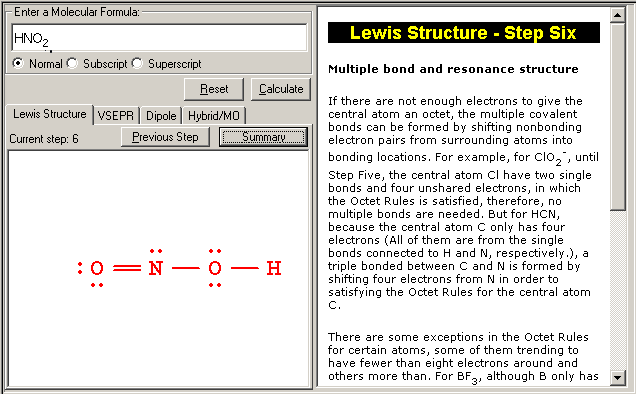
0 thoughts on “Lewis structure generator”