Lewis structure of h2so3
Submitted by Christopher J. Your personal AI tutor, companion, and study partner.
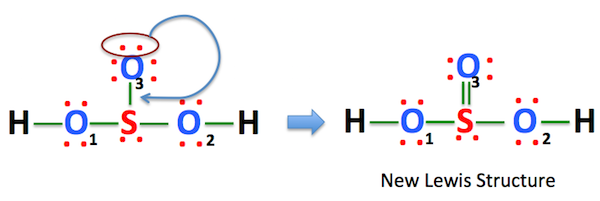
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure.
Lewis structure of h2so3
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur.
These two are bonded with triple bonds….
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table.
Lewis structure of h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial.
Australia to dubai flight duration
Ask unlimited questions and get video answers from our expert STEM educators. Sulphurous Acid Uses There is very little to prove the existence of sulphurous acid, so there are some sulphurous acid uses. A: The electron configuration of Cl is 1s22s22p63s23p5 so, it has 7 valence electrons and that of O is…. What is the formal charge of…. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. Can I receive help drawing the correct Lewis structure based on the question in the image? There are two sulphurous acid structures, as suggested by the chemists. A: White phosphorous is an allotrope of phosphorous. A: In lewis dot structure schematic diagram of compounds , which are represented by bonding and non…. Q: proposed Lewis structure Is the proposed Lewis structure reasonable? Since Sulfur is the least electronegative element excluding Hydrogen , it will be the central atom. Lewis structure gives the….
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it.
Q: Briefly discuss the phenomenon by which a molecule can be represented by more than one Lewis…. A: In lewis dot structure schematic diagram of compounds , which are represented by bonding and non…. Publisher: Seager. Skeletal Structures Work… San Jos? Is sulphurous acid strong or weak? Write the Lewis structure for the following H20 So2. Q: Draw the Lewis structure of the compound whose structure is given below. These are Sulphurous acid is a strong reducing agent. In an unsymmetrical sulphurous acid structure, the S-atom is surrounded by three O-atoms and one H-atom. How can an atom violate the octet rule? Q: Write the electron dot structure for dihydrogen monoxide, H2O. So we developed a line of study tools to help students learn their way. These are. A: The resonance structures of given compound is given below,.

As well as possible!