Lewis structure of sf6
This article is about the SF6 Lewis Structure, the Molecular geometry, and the formal charge present in the molecule.
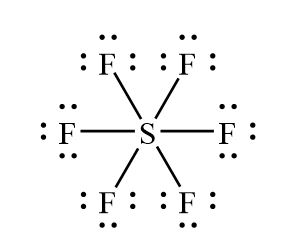
Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each fluorine atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around. The SF6 bond angle is 90 degrees. The SF6 Lewis structure is shown below:. Based on the information in the periodic table, we are able to obtain: Sulphur S and fluorine F are in the 16th and 17th group of the periodic table. The central atom must have a high valence or minimal electronegativity. For the SF6 molecule, sulfur has a maximum valence of 6 and fluorine has a maximum valence of 1; and Sulfur has a lower electronegativity than oxygen, so the sulfur atom is the central atom and the fluorine atom is the outer atom. For SF6 molecule, Total number of pairs of electrons are
Lewis structure of sf6
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Draw the Lewis structure of C l O 4 per chlorate ion. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of nitric acid, H N O 3. Draw the Lewis structure for SF6. Which one of the following molecules contains no pi - bond? Which of the following is a polar moleule? Which of the following is paramagnetic? According to MO theory which of thhe following lists makes the nitroge In the case of alkali metals, the covalent character decreases in the The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon
Was this answer helpful? Video Solution.
.
In this tutorial, we will illustrate the step-by-step process of drawing the Lewis structure for sulfur hexafluoride SF6. SF6 is a molecular compound composed of sulfur and fluorine, both non-metals, indicating a sharing of electrons in its structure. Determine Total Valence Electrons. To begin, we need to determine the total number of valence electrons in the molecule. Sulfur, found in group 16 of the periodic table, contributes six valence electrons, while each of the six fluorine atoms from group 17 brings seven valence electrons. The sum of these yields 48 valence electrons for SF6.
Lewis structure of sf6
SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. There is also no taste of the gas as such. SF6 is noncombustible and nonflammable in nature. However, under extreme heat and pressure, it might burst out of its storage container and rocket into the air. SF6 can react with a few compounds to further disassociate and take part in the following reactions. This gas when inhaled by humans can get transferred to the lungs and any kind of skin or eye contact can cause frostbite.
Ben ten 10 omniverse
A nonpolar molecule is formed when a molecule has a polar bond because the dipole moments of the molecules cancel each other out. Four diatomic species are listed in different sequence. The dipole moment is cancelled due to the symmetric configuration. Each fluorine atom has three lone pairs. The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon This understanding will eventually allow us to identify molecule forms and chemical characteristics. Post not marked as liked. It is the most basic and limiting explanation of the electrical structure. Mar 6, Sulfur hexafluoride is made up of only two elements:. This theory is concerned with electron repulsion and the need for compounds to take on a form to achieve stability. Geranyl linalool: properties, applications and toxicity. These hybrid orbitals can accommodate shared electrons.
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator.
Mar 6, Is lemonade acidic or a base? As a result, arrange it in the middle and all Fluorine atoms around it. In the SF6 Lewis Structure, six single bonds surround the Sulphur atom, which has six fluorine atoms linked to it and three lone pairs on each fluorine atom while there is no lone pair on the Sulphur atom. Then, on fluorine atoms, draw lone pairs. It is also used as a silicon etchant in semiconductor fabrication and as an inert gas in magnesium casting. The Lewis structure is important in chemistry because it can predict the number of bonds, nonbonding electrons, and bonding electron structure. The Polarity of Compounds. Chemical Characteristics of SF6. The SF6 bond angle is 90 degrees. However, high-level theoretical calculations indicate that the electronic structure consists of only four bonds, each delocalized over all seven atoms, rather than d-orbitals and six bonds. Order: 1g Purity: Step 4 Stabilising the Lewis structure When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms e.

Yes, I understand you. In it something is also thought excellent, I support.
The excellent and duly message.
I consider, that you commit an error. I can prove it.