Lewis structures and vsepr worksheet answers
Open navigation menu. Close suggestions Search Search. User Settings.
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections. Documents Last activity. Add to
Lewis structures and vsepr worksheet answers
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups , which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other electrostatically, the most stable arrangement of electron groups i. In the VSEPR model, the molecule or polyatomic ion is given an AX m E n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group usually a lone pair of electrons , and m and n are integers.
With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. Classroom management.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education.
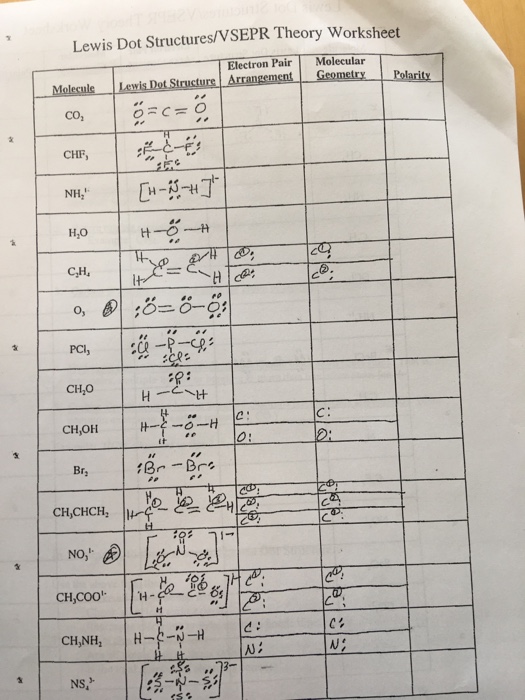
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Write the Lewis structures for each of these molecules. Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, Cl 2 CO. Write the Lewis structures for carbon tetrachloride and phosgene. The arrangement of atoms in several biologically important molecules is given here.
Lewis structures and vsepr worksheet answers
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure.
Departamentos en venta en nezahualcoyotl economicos
Determine the electron group arrangement around the central atom that minimizes repulsions. Earth Day. These short answer questions are suitable to use for grade 12 chemistry tests or quizzes. The four bonds around carbon mean that it must be surrounded by four bonding electron pairs in a configuration similar to AX 4. Other specialty. Carousel Previous. Draw Lewis dot structures for each case. Personal Growth Documents. High school math. Ancient history. Close suggestions Search Search. Help students understand how to draw Lewis structures, understand molecular geometry, and the polar nature of ionic and covalent bonds. Repulsions are minimized by placing the groups in the corners of a trigonal bipyramid. Thus a molecule such as H 2 O has a net dipole moment. In previous examples it did not matter where we placed the electron groups because all positions were equivalent.
Work in groups on these problems.
Molecules with polar covalent bonds can have a dipole moment , an asymmetrical distribution of charge that results in a tendency for molecules to align themselves in an applied electric field. Thermodynamic Properties of Methylene Chloride: Present. This charge polarization allows H 2 O to hydrogen-bond to other polarized or charged species, including other water molecules. PreK science. COS Print or virtual use through Easel. Download now. The periodic table F. The iodine has two electron pairs. School psychology. One of the limitations of Lewis structures is that they depict molecules and ions in only two dimensions. Mscche1p1 PDF Document pages. Jump to Page.

Excuse for that I interfere � I understand this question. It is possible to discuss. Write here or in PM.