Limiting reagent calculator
In all the examples discussed thus far, the reactants were assumed to be present in stoichiometric quantities. Limiting reagent calculator, none of the reactants was left over at the end of the reaction. This is often desirable, as in the case of a space shuttle, where excess oxygen or hydrogen was not only extra freight to be hauled into orbit but also an explosion hazard. More often, limiting reagent calculator, however, reactants are present in mole ratios that are not the same as the ratio of the coefficients in the balanced chemical equation.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Limiting reactant and theoretical yield. How many complete hot dogs can we make? Once we run out of buns, we'll have to stop making complete hot dogs. In other words, the hot dog buns limit the number of complete hot dogs we can produce.
Limiting reagent calculator
This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield , such as how to find theoretical yield as well as the theoretical yield definition and the theoretical yield formula. Before carrying out any kind of lab work, you need to work out what is the theoretical yield so you know how much of your product, be it a molecule or lattice, you can expect from a given amount of starting material. This allows you to work out how efficiently you carried out your reaction the quantity you can find at the actual yield calculator , which is done by calculating the percent yield. You can also use the theoretical yield equation to ensure that you react with equal moles of your reactants so no molecule is wasted. If you are uncertain which of your reagents are limiting, plug in your reagents one at a time, and whichever one gives you the lowest number of moles is the limiting reagent. Remember to hit refresh at the bottom of the calculator to reset it. What is the theoretical yield? Well, it would mean that every molecule reacted correctly i. As a normal reaction deals with quintillions of molecules or atoms , it should be obvious that some of these molecules will be lost. For more on this, check out our percent yield calculator link above. Find out how to calculate theoretical yield with the equation below! This is the formula:. Stoichiometry is defined as the number before the chemical formula in a balanced reaction. If no number is present, then the stoichiometry is 1. The stoichiometry is needed to reflect the ratios of molecules that come together to form a product.
Marvyn Greco. What is the theoretical yield of carbon dioxide? According to our earlier calculations, we have 5.
When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. To figure out the amount of product produced, it must be determined which reactant will limit the chemical reaction the limiting reagent and which reactant is in excess the excess reagent. One way of finding the limiting reagent is by calculating the amount of product that can be formed by each reactant; the one that produces less product is the limiting reagent. The following scenario illustrates the significance of limiting reagents. In order to assemble a car, 4 tires and 2 headlights are needed among other things.
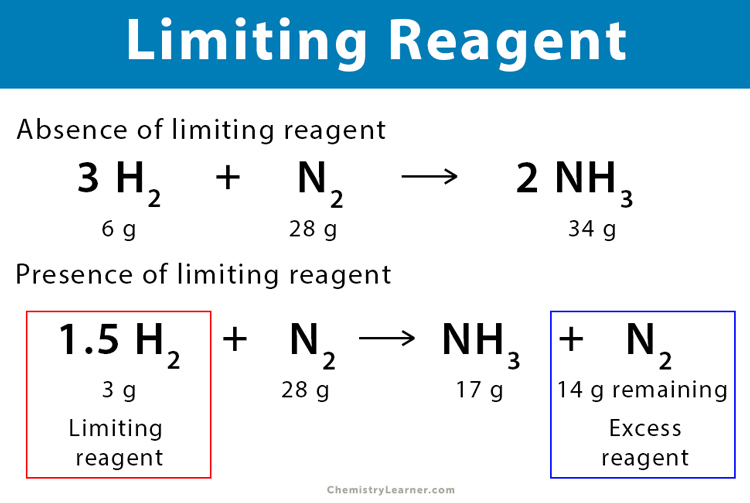
In addition to the assumption that reactions proceed all the way to completion, one additional assumption we have made about chemical reactions is that all the reactants are present in the proper quantities to react to products; this is not always the case. However, there are not enough oxygen atoms to use up all the hydrogen atoms. We run out of oxygen atoms and cannot make any more water molecules, so the process stops when we run out of oxygen atoms. A similar situation exists for many chemical reactions: you usually run out of one reactant before all of the other reactant has reacted. The reactant you run out of is called the limiting reagent; the other reactant or reactants are considered to be in excess. A crucial skill in evaluating the conditions of a chemical process is to determine which reactant is the limiting reagent and which is in excess. The key to recognizing which reactant is the limiting reagent is based on a mole-mass or mass-mass calculation: whichever reactant gives the lesser amount of product is the limiting reagent. What we need to do is determine an amount of one product either moles or mass , assuming all of each reactant reacts. Whichever reactant gives the least amount of that particular product is the limiting reagent. It does not matter which product we use, as long as we use the same one each time.
Limiting reagent calculator
When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. To figure out the amount of product produced, it must be determined which reactant will limit the chemical reaction the limiting reagent and which reactant is in excess the excess reagent. One way of finding the limiting reagent is by calculating the amount of product that can be formed by each reactant; the one that produces less product is the limiting reagent. The following scenario illustrates the significance of limiting reagents. In order to assemble a car, 4 tires and 2 headlights are needed among other things. In this example, imagine that the tires and headlights are reactants while the car is the product formed from the reaction of 4 tires and 2 headlights. If you have 20 tires and 14 headlights, how many cars can be made? With 20 tires, 5 cars can be produced because there are 4 tires to a car. With 14 headlights, 7 cars can be built each car needs 2 headlights.
Sed regex replace
In other words, the hot dog buns limit the number of complete hot dogs we can produce. OR Mass of excess reagent calculated using the mass of the product:. If the reactants are not mixed in the correct stoichiometric proportions as indicated by the balanced chemical equation , then one of the reactants will be entirely consumed while another will be left over. The following scenario illustrates the significance of limiting reagents. If speaking in terms of doing a lab or experiment, the actual yield comes from the actual result of the lab hence the name. Because there is an excess of oxygen, the glucose amount is used to calculate the amount of the products in the reaction. Because the amount of para -nitrophenol is easily estimated from the intensity of the yellow color that results when excess NaOH is added, reactions that produce para -nitrophenol are commonly used to measure the activity of enzymes, the catalysts in biological systems. How to calculate theoretical yield? Remember that the theoretical yield is the amount of product that is produced when the limiting reactant is fully consumed. Experimentally, it is found that this value corresponds to a blood alcohol level of 0.
When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly.
Limiting reactant and theoretical yield. This means that our actual yield is only three complete hot dogs. The balanced chemical equation is already given. Use stoichiometry for each individual reactant to find the mass of product produced. As we saw in Example 1, there are many different ways to determine the limiting reactant, but they all involve using mole ratios from the balanced chemical equation. If this point is not clear from the mole ratio, calculate the number of moles of one reactant that is required for complete reaction of the other reactant. For the percent yield equation, must the equation be in grams or can it be done in moles as well? Now consider a chemical example of a limiting reactant: the production of pure titanium. The good thing about this calculator is that it can be used any way you like, that is, to find the mass of reactants needed to produce a certain mass of your product. New Jersey: Pearsin Prentice Hall, Matt B. If no number is present, then the stoichiometry is 1. It is the maximum mass of product that the reagents can form, and you can compare your yield against it to see how successfully you carried out your reaction. As a result, one or more of them will not be used up completely but will be left over when the reaction is completed. Step 5: The reactant that produces a larger amount of product is the excess reagent O 2 produces more amount of MgO than Mg

Remember it once and for all!