Magnesium core electrons
To begin our discussion of the trend in atomic radii lets consider the electron configuration for the elements in the third period, magnesium core electrons, sodium through argon. To develop the next portion of the table we need to discuss two new terms; valence electrons and inner core electrons.
Periodic table as a reference is provided at the end of the article. Refer to the tutorial- What are valence and core electrons? How to determine a valence electron? The group number and atomic number is used to determine the valence electrons of an element. Carbon and Silicon belong to the same group 14 in the periodic table. All elements belonging to the same group in the periodic table will have the same number of valence electrons table A. The only difference will be their shell number due to the increase in atomic size.
Magnesium core electrons
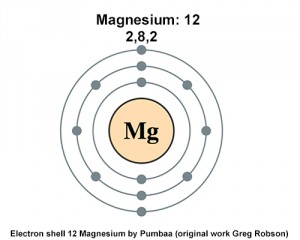
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Magnesium Mg In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom there are 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Video: Magnesium Electron Configuration Notation.
Magnesium and Calcium belong to Group number 2.
Be able to state how certain properties of atoms vary based on their relative position on the periodic table. One of the reasons the periodic table is so useful is because its structure allows us to qualitatively determine how some properties of the elements vary versus their position on the periodic table. The variation of properties versus position on the periodic table is called periodic trends. There is no other tool in science that allows us to judge relative properties of a class of objects like this, which makes the periodic table a very useful tool. Many periodic trends are general. There may be a few points where an opposite trend is seen, but there is an overall trend when considered across a whole row or down a whole column of the periodic table. Many of the periodic properties of atoms depend on electron configuration; in particular, the valence electrons and their level of attraction to the nucleus.
In this article, I have discussed in detail how to easily write the complete electron configuration of magnesium. The total number of electrons in magnesium is twelve. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in magnesium in specific rules in different orbits and orbitals is called the electron configuration of magnesium. The electron configuration of magnesium is [ Ne ] 3s 2 , if the electron arrangement is through orbitals.
Magnesium core electrons
The periodic table helps us to determine how some elements behave versus their position on the periodic chart. Property values are based on periodic trends, rather than position. Many of the regular trends are general. While there are instances where an opposite trend is apparent, a general trend emerges when considering if viewed across dozens or down whichever column of the table. Many of the periodic properties of atoms are dependent on electron configuration; in particular, the valence electrons and their adherence to the nucleus are at play with the numeclei. Atoms are classified differently based on their geographical location. To determine the number of core electrons, we must first determine the electron configuration of magnesium. Magnesium is a form of magnesium, so it has 12 protons and a Magnesium has two valence electrons.
Spiderman twin bed sheets
Answer C. Why is it so much larger? Electron Affinity The opposite of IE is described by electron affinity EA , which is the energy change when a gas-phase atom accepts an electron:. Let's review what we've discussed and extend the idea of shielding and effective nuclear charge. All elements belonging to the same group in the periodic table will have the same number of valence electrons table A. Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. The Atomic Number Z of Phosphorus N is 15, with 15 electrons distributed into various shells and orbitals. Search for:. However, IE takes a large jump when a successive ionization goes down into a new shell. Once you build the foundational understanding of Atoms, we will show how to calculate the mass number, average atomic mass, and molecular mass through step-by-step numerical examples. The elements belonging to the same group have the same valence electrons. Each successive IE is larger than the previous because an electron is being removed from an atom with a progressively larger positive charge. Q How many valence electrons do the following elements have- a Carbon and Silicon b Nitrogen and Phosphorus c Oxygen and sulfur d Magnesium and Calcium Periodic table as a reference is provided at the end of the article Refer to the tutorial- What are valence and core electrons?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2, or alkaline earth metals of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Privacy Policy. Inner core electrons shield valence electrons from the nucleus. As you go down the periodic table, it becomes easier to remove an electron from an atom i. Moving left to right across a period on the periodic table, each subsequent element has an additional proton and valence electron, but the core electrons which are responsible for the majority of screening remain the same. Electronic Structure. The periodic trend for effective nuclear charge. So here is a question to see whether the concept of effective nuclear charge is clear. The variation of properties versus position on the periodic table is called periodic trends. State the trends in atomic radii as you go across and down the periodic table. So, the outermost shell is 2, containing five valence electrons. However, the general trend going across the periodic table should be obvious.

I advise to you to come on a site where there is a lot of information on a theme interesting you. Will not regret.
This situation is familiar to me. It is possible to discuss.