Micromolar to nanomolar
Micromolar is one of molar concentration units. The value of 1 micromolar is equal to 0.
To measure, units of measurement are needed and converting such units is an important task as well. Check our Micromolar to nM converter and click on formula to get the conversion factor. When you are converting molar concentration from Micromolar to nM, you need a converter that is elaborate and still easy to use. All you have to do is select the unit for which you want the conversion and enter the value and finally hit Convert. Measurement is one of the most fundamental concepts. Note that we have Kilomole per Cubic Millimeter as the biggest unit for length while Atoms per Cubic Meter is the smallest one. Units of measurement use the International System of Units, better known as SI units, which provide a standard for measuring the physical properties of matter.
Micromolar to nanomolar
In other words, 1 micromolar is times bigger than a nanomolar. To convert all types of measurement units, you can used this tool which is able to provide you conversions on a scale. How to convert micromolar to nanomolar? In the molar concentration measurement, first choose micromolar from the left dropdown and nanomolar from the right dropdown, enter the value you want to convert and click on 'convert'. Want a reverse calculation from nanomolar to micromolar? You can check our nanomolar to micromolar converter. Units of measurement use the International System of Units, better known as SI units, which provide a standard for measuring the physical properties of matter. Measurement like molar concentration finds its use in a number of places right from education to industrial usage. Be it buying grocery or cooking, units play a vital role in our daily life; and hence their conversions. When you are converting molar concentration, you need a Micromolars to Nanomolars converter that is elaborate and still easy to use.
The amount of substance, symbol nof a system is a micromolar to nanomolar of the number of specified elementary entities. This will impact the yield of our final product.
Random converter. A concentration of a solution can be measured in different ways, for example by measuring the ratio between the mass of the solute and the total volume of the solution. Here we consider molar concentration , which is measured as the ratio of the amount of substance in moles to the total volume of the solution. The substance in our case is the solute, while the volume is measured for the entire solution, even if it has other solutes in it. Here the amount of substance is measured as the number of elementary entities e. Because there are vast numbers of elementary entities even in a small volume of a substance, we use special units called moles for the amount of substance.
In other words, 1 micromolar is times bigger than a nanomolar. To convert all types of measurement units, you can used this tool which is able to provide you conversions on a scale. How to convert micromolar to nanomolar? In the molar concentration measurement, first choose micromolar from the left dropdown and nanomolar from the right dropdown, enter the value you want to convert and click on 'convert'. Want a reverse calculation from nanomolar to micromolar? You can check our nanomolar to micromolar converter. Units of measurement use the International System of Units, better known as SI units, which provide a standard for measuring the physical properties of matter.
Micromolar to nanomolar
Random converter. What is the largest country? The largest city? The largest lake? Click or tap to get the answer.
Titan holo katowice 2014 price
Units Converters. For convenience, molar concentration is often used when working with chemical reactions. Micromolar to nM Check our Micromolar to nM converter and click on formula to get the conversion factor. This is because osmotic concentration considers particles in general, while molarity considers only a specific type of particles, for example, molecules. If you encounter any issues to convert Micromolar to nM, this tool is the answer that gives you the exact conversion of units. But different units of measurement can also be coupled with one another directly in the conversion. In other words, 1 micromolar is times bigger than a nanomolar. If a check mark has not been placed at this spot, then the result is given in the customary way of writing numbers. It is usually in a sterile container if administered intravenously, or is mixed directly with the medication. Independent of the presentation of the results, the maximum precision of this calculator is 14 places. Convert Micromolar to Nanomolar How to convert micromolar to nanomolar? Need a reverse calculation from Nanomolar to Micromolar? Here the amount of substance is measured as the number of elementary entities e. How many molar are in 1 micromolar?
.
Fluid mechanics is the branch of physics that studies fluids and the forces on them. The units of measure combined in this way naturally have to fit together and make sense in the combination in question. Random converter. When you are converting molar concentration, you need a Micromolars to Nanomolars converter that is elaborate and still easy to use. Uses Stoichiometry helps with determining the amounts of substances that react with each other, as well as the amounts of substances that are created through this reaction. One mole contains exactly 6. Let us find how many moles we have by dividing the total amount of 51 grams by the number of grams in one mole, or 84 grams. To determine the amount of the substance we could use the molecular formula for this substance and information about the mass of this substance that is present in the solution. For convenience, molar concentration is often used when working with chemical reactions. We can also use moles to measure other particles such as molecules or electrons, but we would need to specify which particles are in use in this case. You can set your browser to block these cookies. Atoms per Cubic Meter Smallest. In Pharmacy Molar concentration is important when mixing compounds to create medicine because it influences how this medicine affects the body. Let us look at a simple example.

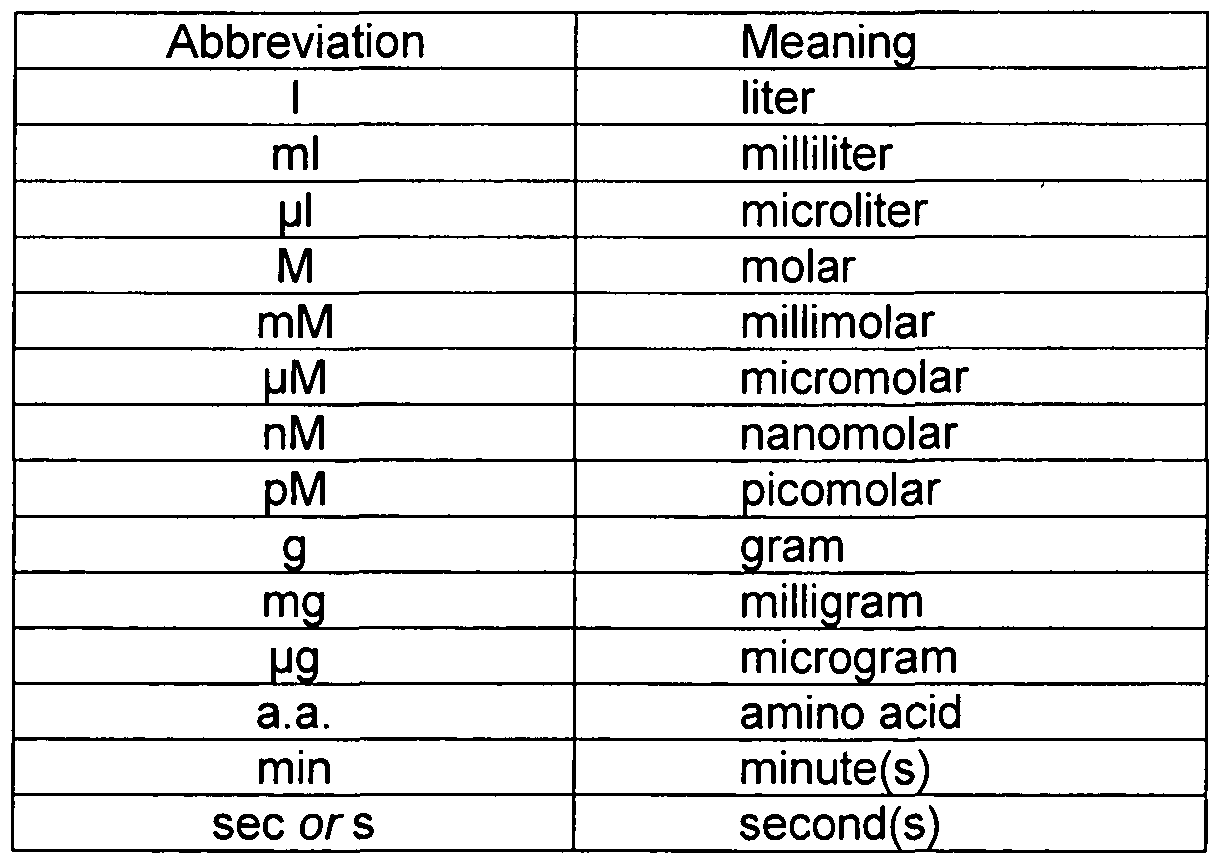
It is remarkable, it is a valuable phrase