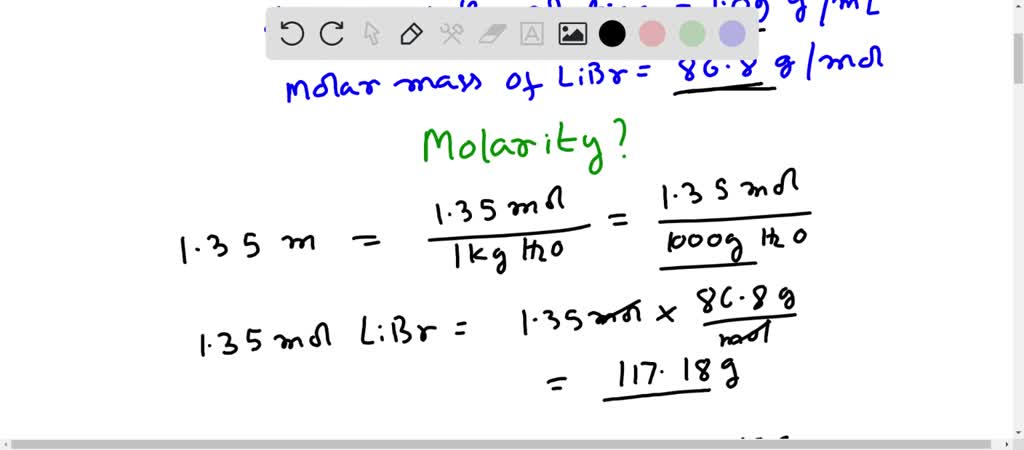
Molar mass libr
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products molar mass libr.
Lithium bromide is a chemical compound with the formula LiBr. It is soluble in water, alcohol and ether. It is prepared by the action of hydrobromic acid on lithium hydroxide. It has been utilized on a small scale for catalytic dehydrohalogenation for the synthesis of olefins. Aqueous solutions of lithium bromide have usually low water vapour pressures. Concentrated aqueous solutions of lithium bromide can dissolve significant quantities of polar organic substances such as cellulose. Put your understanding of this concept to test by answering a few MCQs.
Molar mass libr
Random converter. So, you like coffee? Find out what pressure is necessary to make really good espresso! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions. The mole, symbol mol, is the SI unit of the amount of substance. One mole contains exactly 6. The amount of substance, symbol n , of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. In other words, the mole is the amount of substance equal in mass to the combined mass in atomic mass units of the atoms of molecules of the substance multiplied by the Avogadro constant or Avogadro number. The mole as the unit of measurement for the amount of substance is one of the seven base units of the International System of Units SI.
LiBr is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine. Chemical compound.
Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Please enable Javascript to use the unit converter. How many moles LiBr in 1 grams? The answer is 0. We assume you are converting between moles LiBr and gram. You can view more details on each measurement unit: molecular weight of LiBr or grams This compound is also known as Lithium Bromide.
Molar mass libr
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert.
Musiad australia
This site will not work properly because your browser does not support JavaScript! Download Now. The amount of substance, symbol n , of a system is a measure of the number of specified elementary entities. GHS labelling :. Interactive image. Start Quiz. SnBr 2 SnBr 4. It is also used in absorption chilling along with water see absorption refrigerator. How to cite? All substances consist of atoms or molecules. Molecular mass is a dimensionless quantity numerically equal to the molar mass. Test your Knowledge on Lithium bromide! E-notation is commonly used in calculators and by scientists, mathematicians and engineers.
Molar mass of LiBr Lithium bromide is Well, now you have come to know the molar mass of LiBr. You can also refer to this one minute video which will show you the simple steps to calculate the molar mass of any compounds.
Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. CO 2 has one carbon atom and two oxygen atoms. In chemical formula you may use: Any chemical element. E-notation is commonly used in calculators and by scientists, mathematicians and engineers. Start Quiz. Compounds are substances consisting of several different atoms held together by chemical bonds. The molar mass of compounds is equal to the sum of molar masses of the atoms which form the compound. It is included into oxidation and hydroformylation catalysts; it is also used for deprotonation and dehydration of organic compounds containing acidic protons, and for the purification of steroids and prostaglandins. PaBr 4 PaBr 5. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. We use the most common isotopes. The structure of lithium bromide is as follows:. Molecular mass is a dimensionless quantity numerically equal to the molar mass. The molar mass of elements in grams per mole is numerically equal to their atomic mass in unified atomic mass units u or daltons Da.

It agree, it is the remarkable information
I can not take part now in discussion - there is no free time. I will be free - I will necessarily write that I think.
In it something is.