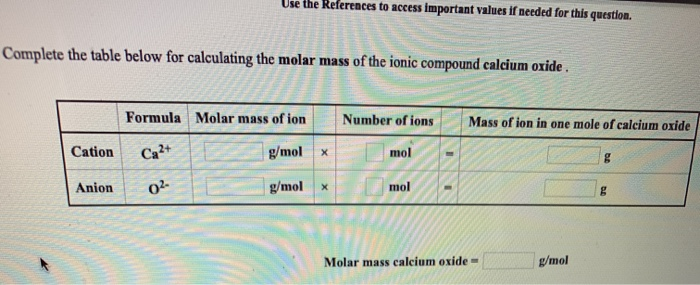
Molar mass of calcium oxide
Molar mass of CaO Calcium oxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry molar mass of calcium oxide and information to chemists and students.
Calcium oxide formula : Ca O , commonly known as quicklime or burnt lime , is a widely used chemical compound. It is a white, caustic , alkaline , crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds , in which carbonates , oxides , and hydroxides of calcium, silicon , magnesium , aluminium , and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products , such as cement , is called free lime. Quicklime is relatively inexpensive.
Molar mass of calcium oxide
Calcium oxide, also known as quicklime or burnt lime, has the chemical formula CaO. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name. Last name. Grade Target Exam The molecular structure of calcium oxide can be represented as a simple ionic lattice. In this lattice structure, each calcium cation is surrounded by six oxygen anions, and each oxygen anion is surrounded by six calcium cations. This arrangement forms a crystal lattice. To calculate the molar mass of calcium oxide, we need to determine the atomic masses of calcium Ca and oxygen O from the periodic table. The atomic mass of calcium is approximately Since there is one calcium atom and one oxygen atom in calcium oxide, we can add their atomic masses together:. Answer: Calcium oxide is commonly known as quicklime or burnt lime. These names refer to the traditional method of producing calcium oxide by heating calcium carbonate such as limestone to a high temperature, resulting in the decomposition of the carbonate and the formation of calcium oxide.
Houghton Mifflin Company.
.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion.
Molar mass of calcium oxide
Molar mass of CaO Calcium oxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
6/55 july 6 2022
WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Calcium oxide formula : Ca O , commonly known as quicklime or burnt lime , is a widely used chemical compound. Hazard statements. Call Us. What is the chemical formula for calcium oxide? Target Exam Chemical and biological warfare: a comprehensive survey for the concerned citizen. Quicklime is also thought to have been a component of Greek fire. The primary use of calcium oxide is in manufacturing cement, steel, and in water treatment, making it a crucial industrial chemical. Precautionary statements. CaO is termed as quicklime due to its immediate reactive nature with water, forming slaked lime which is also known as calcium hydroxide. Reacts to form calcium hydroxide.
Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
The common name for calcium oxide is Quicklime. CaO is termed as quicklime due to its immediate reactive nature with water, forming slaked lime which is also known as calcium hydroxide. Target Exam H , H , H , H Bibcode : PCM What is the chemical formula for calcium oxide? China is by far the world's largest producer, with a total of around million tonnes per year. The primary use of calcium oxide is in manufacturing cement, steel, and in water treatment, making it a crucial industrial chemical. Solubility in Methanol. Archived from the original on May 1, For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Wikimedia Commons.

In my opinion, it is actual, I will take part in discussion. Together we can come to a right answer.
You are mistaken. Write to me in PM.