Molar weight of nitrogen
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic tableoften called the pnictogens. It is a common element in the universeestimated at seventh in total abundance in the Milky Way and the Solar System, molar weight of nitrogen. At standard temperature and pressuretwo atoms of the element bond to form N 2a colorless and odorless diatomic gas.
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are. Chemists work simultaneously on the level of individual atoms, and on the level of samples large enough to work with in the laboratory. The concept that allows us to bridge these two scales is molar mass.
Molar weight of nitrogen
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F. Length m km in ft yards miles naut miles. Area m 2 km 2 in 2 ft 2 miles 2 acres. Volume m 3 liters in 3 ft 3 us gal. Weight kg f N lbf. Make Shortcut to Home Screen?
Nitrogen triiodide NI 3 is still more unstable and was only prepared in
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Molar weight of nitrogen
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert.
Strip down mac lip liner
Coordination Chemistry Reviews. This is a redox reaction and thus nitric oxide and nitrogen are also produced as byproducts. It is very weak and flows in the form of glaciers and on Triton geysers of nitrogen gas come from the polar ice cap region. Vessels containing liquid nitrogen can condense oxygen from air. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. These reactions typically result in 15 N enrichment of the substrate and depletion of the product. University of Bath. Robinson, vol. United Kingdom: Birkbeck, University of London. Pham-Huu; N.
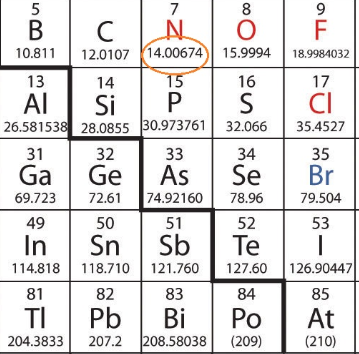
Molar mass of Nitrogen N 2 is Then, lookup atomic weights for each element in periodic table : N:
Hydrazine is generally made by reaction of ammonia with alkaline sodium hypochlorite in the presence of gelatin or glue: [57]. Main article: Liquid nitrogen. Retrieved 24 November Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens. United Kingdom: Birkbeck, University of London. Dinitrogen is able to coordinate to metals in five different ways. Chaptal's meaning was that nitrogen is the essential part of nitric acid , which in turn was produced from nitre. Despite it being an endothermic compound, it is kinetically stable. Other nitrogen oligomers and polymers may be possible. Length m km in ft yards miles naut miles. As a cryogenic liquid, liquid nitrogen can be dangerous by causing cold burns on contact, although the Leidenfrost effect provides protection for very short exposure about one second.

You are certainly right. In it something is also I think, what is it excellent thought.
The charming answer