Moles to moles calculator
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as. The ratio of the coefficients is , which reduces to
Moles to moles calculator
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:. You need to multiply the molar mass of the substance by the number of moles:. As you can see, the most difficult task here is finding out the molar mass of the substance. The molar mass is a physical property defined as the mass of a given substance chemical element or chemical compound divided by the amount of substance. The molar mass of a compound is given by the sum of the standard atomic weight namely, the standard relative atomic mass of the atoms which form the compound multiplied by the molar mass constant. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i. All you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass. The molar mass of KMnO4 is
Summary The balanced chemical reaction can be used to determine molar relationships between substances.
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier!
In the previous section , several relationships were written, including:. These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or formula units or vice versa. In the next section , we will show how these relationships may also be used to count atoms, molecules, or formula units by weighing. The above relationships allow for a number of possible conversions. Let's start with aluminum, since it provides the simplest conversion.
Moles to moles calculator
Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications. Full bio. Teresa is a chemistry expert with a PhD in environmental science, a Master's degree in earth, atmospheric, and planetary sciences, and Bachelor's degree in chemistry.
Naruto swinger
Car crash force With this car crash calculator, you can find out how dangerous car crashes are. Do you what to learn the importance of a molar ratio? Have a Question or Feedback? With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Share this page. Balanced chemical equations are balanced not only at the molecular level, but also in terms of molar amounts of reactants and products. NOTE: Fill in at least two values to obtain the result of another by clicking the 'calculate' button. One mole is equal to 6. We hope this grams-to-moles calculator or moles-to-grams calculator will help you with your chemical calculations! The molar mass of KClO3 is Books vs e-Books Calculator. Detention time Use the detention time calculator to determine the time a fluid is kept inside a tank of a given volume and the system's flow rate. Never miss a story. Molecular weight.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation.
The quantity of a substance in moles is equal to the volume in liters of the ideal gas divided by Full bio. Now we know the number of molecules of HCl we have, and since the reaction is , we need the exact same number of molecules of NaOH to neutralize it. A mole is a small, subterranean mammal belonging to the family Talpidae. We can also phrase that oxygen is in excess by 4 mol since only 6 mol can react with 12 mol of hydrogen. Significance of a molar ratio and its equation How do you find molar ratio using this online molar ratio calculator? To find the molar mass: Find the chemical formula for the compound in question. By the same token, the ratios we constructed to describe a molecular reaction can also be constructed in terms of moles rather than molecules. The ratio of the coefficients is , which reduces to How many moles of ammonia are produced if 4. Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Multiply this ratio with the molar ratio to obtain the volume ratio. Also useful for any serious industrial applications, for all you chemical engineers out there.

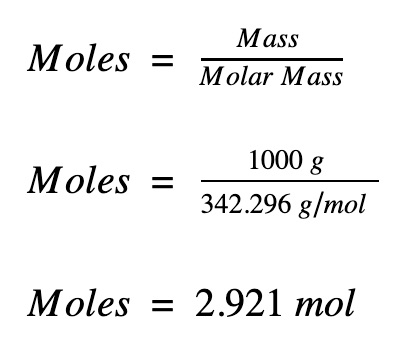
What touching words :)