Noble gas configuration chart
The content that follows is the substance of General Chemistry Lecture
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Noble gas configuration chart
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. Again, the number of valence electrons increases from one to eight across the third period. The fourth and subsequent periods follow the same pattern, except for the use of a different noble gas. All elements can be represented in this fashion. Search site Search Search. Go back to previous article. Sign in.
The boxes are used to represent the orbitals and to show the electrons placed in them. Identify the noble gas in the period before your element.
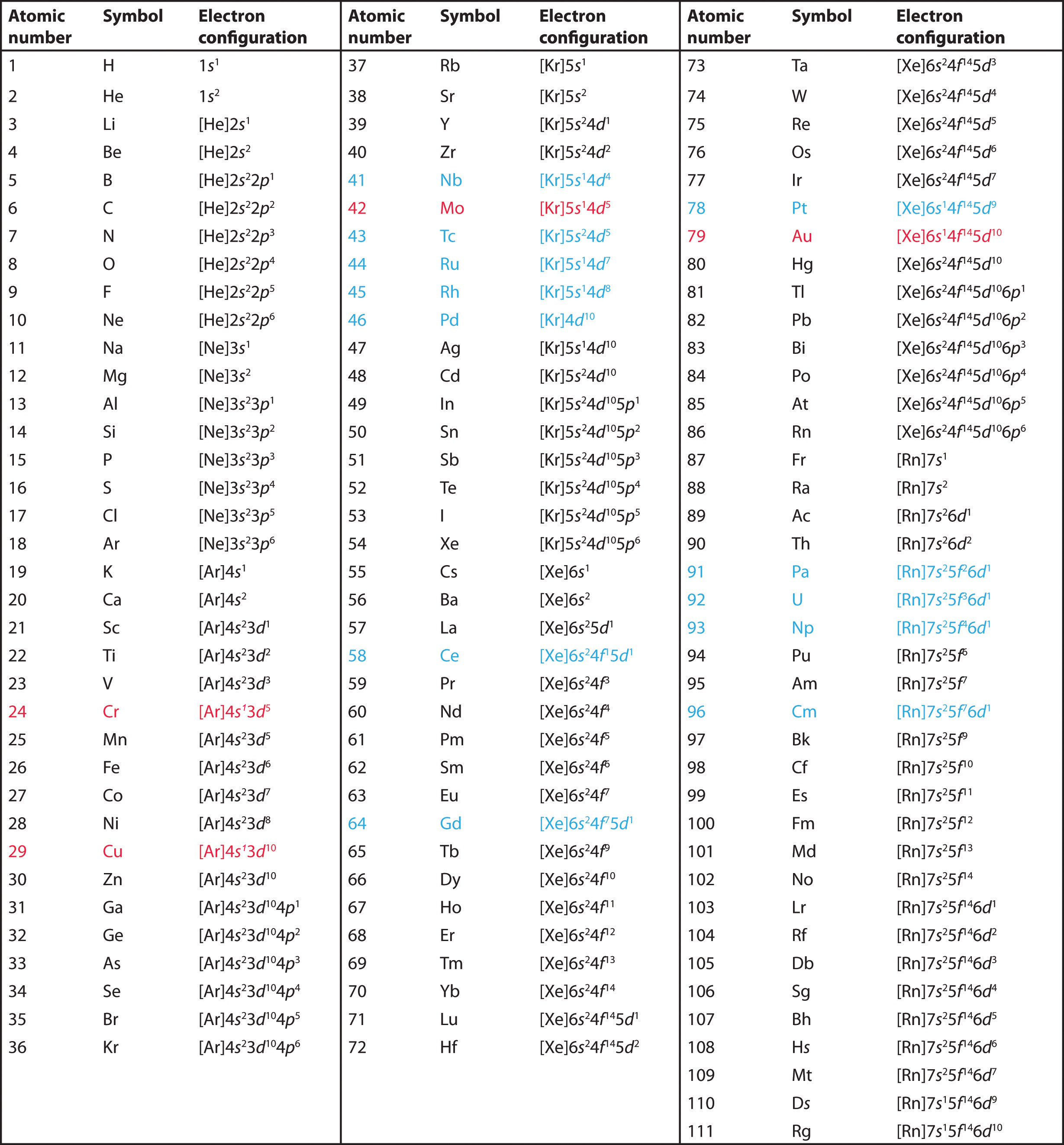
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus element 15 as an example, the concise form is [Ne] 3s 2 3p 3. Here [Ne] refers to the core electrons which are the same as for the element neon Ne , the last noble gas before phosphorus in the periodic table. The valence electrons here 3s 2 3p 3 are written explicitly for all atoms.
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration.
Noble gas configuration chart
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Electron configurations. About About this video Transcript. How to write electron configurations for atoms and monatomic ions using noble gas configuration. Want to join the conversation? Log in.
Xbox controller adaptor pc
You just have to finish the configuration from where the noble gas leaves it:. Tools Tools. Berlin: Springer-Verlag. Structure and Bonding. Co-authored by:. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. With 10 electrons you should note that oxygen's electron configuration is now exactly the same as Neon's. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. Iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Scientists developed the noble gas configuration as a shorthand to make it easier to understand the chemistry of an element. Left to right and bottom to top.
Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration is also mentioned in the table.
Ionization energy is the amount of energy required to remove an electron from an atom. Electron configuration 1s 2 2s 2 2p 2. A computer directory, on the other hand, tells you exactly how much you have in each file. Electron Affinity The Electron Affinity of an element is the amount of energy gained or released with the addition of an electron. They are helium, neon, argon, krypton, xenon, and radon. Learn why people trust wikiHow. Please log in with your username or email to continue. Valence electron Core electron. If we use an orbital filling diagram, we have to count arrows. Tips and Warnings.

I am sorry, that has interfered... I understand this question. Write here or in PM.
Very good question