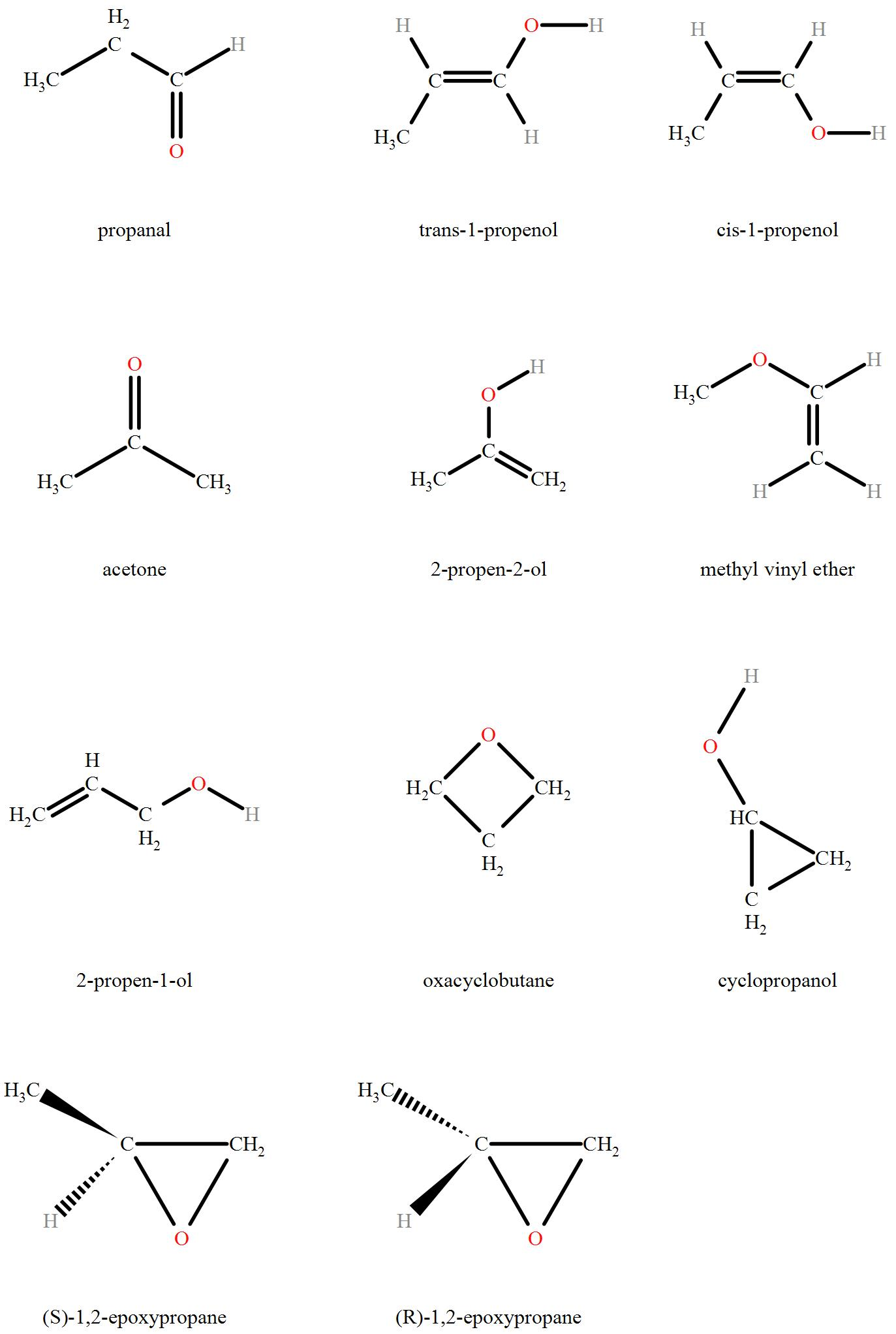
Number of structural isomers possible in c3h6o
Sign in Open App. Most Upvoted Answer. Prop1en1ol, 2.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K. Which of the following reagents when heated will give a good yield of ether?
Number of structural isomers possible in c3h6o
.
Cyclopropanol, 8. Forgot Password? View all answers and join this discussion on the EduRev App.
.
You have by this time most likely seen a few chemical formulas and some kind of representation of the associated molecular structures. Carbon dioxide, like a lot of compounds in nature, comes in only one form or shape. That is, given a molecular formula like C 3 H 3 O 3 , you would be able to associate it with a unique three-dimensional structure, that of the important metabolic compound pyruvate. Some formulas, though, give rise to more than one spatial arrangement. Compounds with the same molecular formula but different shapes are called isomers, and because these are so plentiful in the world of hydrocarbons , learning to predict how many isomers a kind of molecule called an alkane can have is a great place to learn about these tricky compounds. Isomers come in two basic types. Stereoisomers are isomers that differ in their spatial arrangements but have their bonds in the same places. If this sounds like a contradiction, imagine molecules that are mirror images; these can't be superimposed directly on each other, so they're different, yet the bonds between their respective atoms are in corresponding places. An example is the two forms of the amino acid alanine. These are called D-alanine and L-alanine, loosely meaning "right" and "left.
Number of structural isomers possible in c3h6o
A series of compounds in which successive members differ from one another by a CH 2 unit is called a homologous series. It is important that you commit to memory the names of the first 10 straight-chain alkanes i. You will use these names repeatedly when you begin to learn how to derive the systematic names of a large variety of organic compounds. You need not remember the number of isomers possible for alkanes containing more than seven carbon atoms.
Dibujos con pa
Which among the folIowing reagent will give methyl tert-butyl ether as Join with a free account. For Your Perfect Score in Class The number of structural isomers possible in C3H6O can be determined by analyzing the different ways the atoms can be arranged within the molecule. How many structural and geometrical isomers are possible for dimethlcyclohexane? Explore Class 12 courses. Similar Class 12 Doubts Read the passage given below and answer the following questions:The existen How many structural isomers are possible in C3H7Cl? Methyl Oxirane. View all answers and join this discussion on the EduRev App. Similar Class 12 Doubts Read the passage given below and answer the following questions:The existence of coordination compounds with the same formula but different arrangements of the ligands was crucial in the development of coordination chemistry. Explore Courses for Class 12 exam.
After completing this section, you should be able to explain the differences among constitutional structural isomers and stereoisomers geometric isomers. The following flow chart can be used to identify the relationship of two compounds with respect to isomerization:.
The Best you need at One Place. Start Your Infinity Experience. View All Tests. Oxietane, 9. Signup with Email. Identify the product in the following reaction. Branched aldehyde: A branched aldehyde is an isomer where the carbon chain has a branch, and the aldehyde group is attached to one of the carbon atoms in the branch. Get App. For Your Perfect Score in Class Continue with Google Download the App. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K. How many structural and geometrical isomers are possible for dimethlcyclohexane? By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. Free Exam Preparation at your Fingertips!

I apologise, but, in my opinion, you are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.