Otf chemistry
With an accout for my.
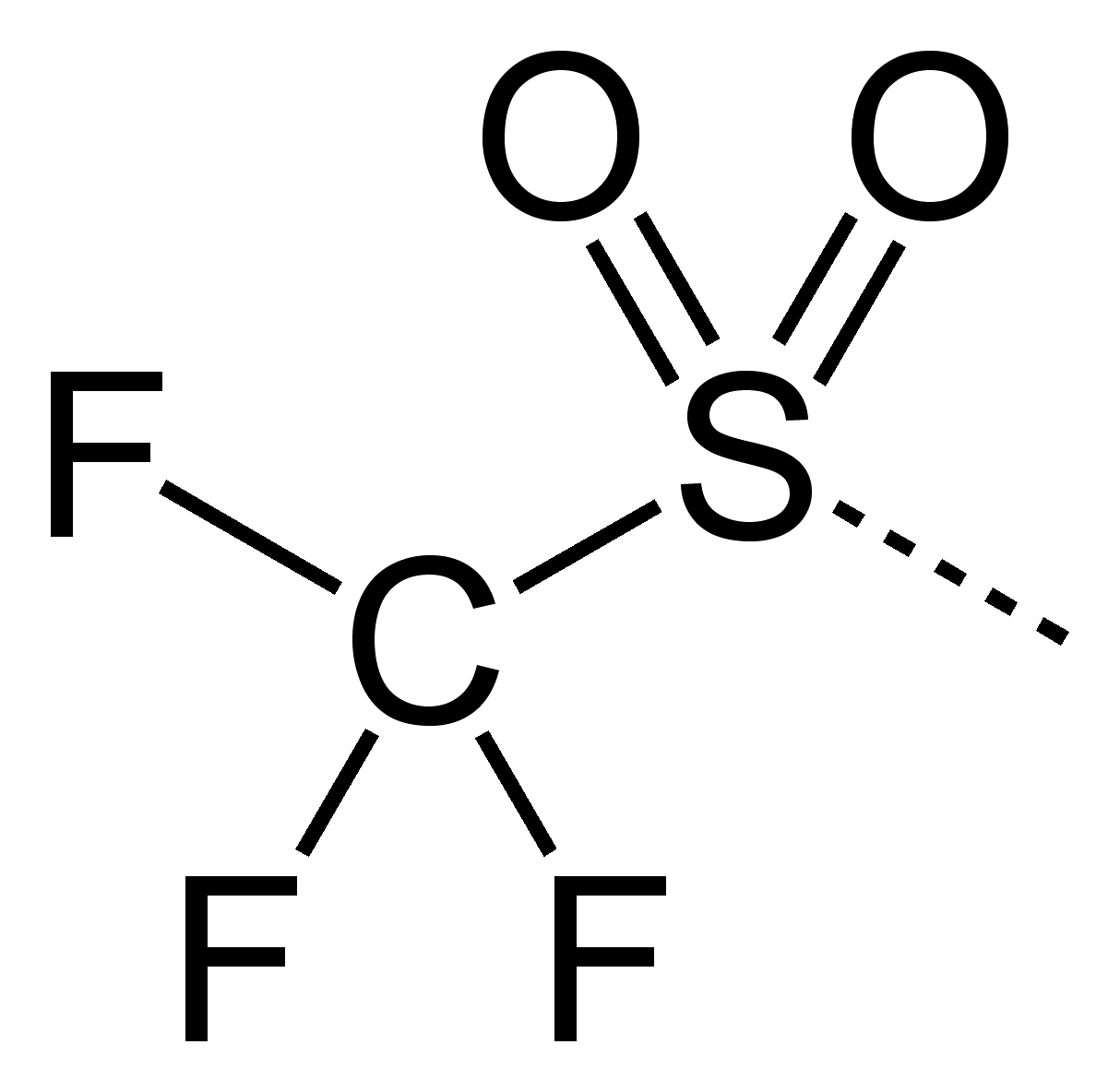
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides. Lithium triflates are used in some lithium ion batteries as a component of the electrolyte.
Otf chemistry
.
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitutionSuzuki couplings and Heck reactions, otf chemistry. My watch list My saved searches My saved topics My newsletter Otf chemistry free of charge.
.
It is a colorless moisture-sensitive liquid. It is the trifluoromethanesulfonate derivative of trimethylsilyl. It is far more electrophilic than trimethylsilyl chloride. Related to its tendency to hydrolyze, tmsOTf is effective for silylation of alcohols : [2]. It was also used in Takahashi Taxol total synthesis and in chemical glycosylation reactions. Trimethylsilyl trifluoromethanesulfonate has a variety of other specialized uses. Deprotection of Boc -protected amines can be achieved using trimethylsilyl trifluoromethanesulfonate and triethylamine or 2,6-lutidine.
Otf chemistry
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
55 second timer
Read Edit View history. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Additional recommended knowledge. A related popular catalyst scandium triflate is used in such reactions as aldol reactions and Diels—Alder reactions. Inorganic Syntheses 28 : European Journal of Organic Chemistry. Lawrance, Peter A. ISBN Journal of the American Chemical Society. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. To use all the functions on Chemie. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate , or from reacting barium triflate with metal sulfates in water: [1]. Download as PDF Printable version. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water.
Remember when discussing the substitution reactions, we said that the hydroxide ion — OH is a poor leaving group since it is quite a strong base. So, to perform a substitution reaction on an alcohol, the hydroxyl group must be first converted into a good leaving group. This can be, for example, a halogen which leaves as a halide ion in substitution reactions.
Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable in water such as aluminum chloride. A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Dixon, Geoffrey A. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. To top. Toggle limited content width. PMID In other projects. My watch list my. It uses material from the Wikipedia article "Triflate". Contents move to sidebar hide. Inorganic Syntheses. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. Triflate , more formally known as trifluoromethanesulfonate , is a functional group with the formula CF 3 SO 3 -. Wikimedia Commons has media related to Triflates.

I apologise, but, in my opinion, you are mistaken. I can defend the position. Write to me in PM.
Unfortunately, I can help nothing, but it is assured, that you will find the correct decision. Do not despair.
I apologise, but, in my opinion, you are not right. Let's discuss it. Write to me in PM.