Peroxynitrite
Federal government websites often end in. The site is secure, peroxynitrite. Peroxynitrite is the product of the diffusion-controlled reaction of nitric oxide and superoxide radicals. Peroxynitrite, a reactive short-lived peroxide with a p K peroxynitrite of 6.
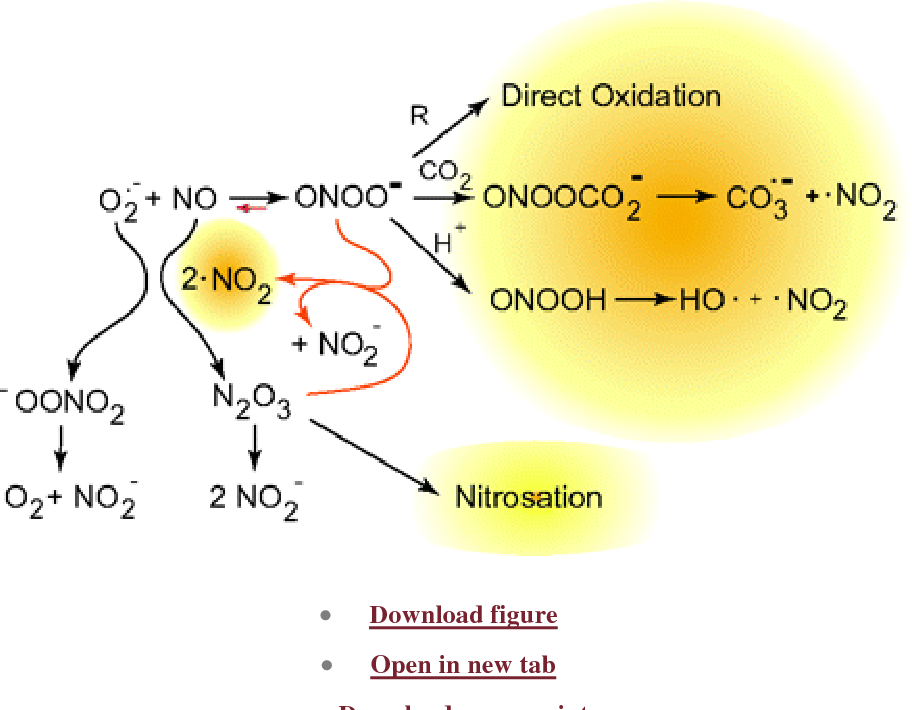
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide : [1] [2] [3]. It is prepared by the reaction of hydrogen peroxide with nitrite : [4]. It is reactive toward DNA and proteins. It is these radicals carbonate radical and nitrogen dioxide that are believed to cause peroxynitrite-related cellular damage. Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions. Contents move to sidebar hide. Article Talk.
Peroxynitrite
.
This reaction was later shown in mammalian peroxiredoxin systems
.
Federal government websites often end in. The site is secure. The discovery that mammalian cells have the ability to synthesize the free radical nitric oxide NO has stimulated an extraordinary impetus for scientific research in all the fields of biology and medicine. Since its early description as an endothelial-derived relaxing factor, NO has emerged as a fundamental signaling device regulating virtually every critical cellular function, as well as a potent mediator of cellular damage in a wide range of conditions. Recent evidence indicates that most of the cytotoxicity attributed to NO is rather due to peroxynitrite, produced from the diffusion-controlled reaction between NO and another free radical, the superoxide anion. Peroxynitrite interacts with lipids, DNA, and proteins via direct oxidative reactions or via indirect, radical-mediated mechanisms.
Peroxynitrite
Federal government websites often end in. The site is secure. Peroxynitrite is the product of the diffusion-controlled reaction of nitric oxide and superoxide radicals. Peroxynitrite, a reactive short-lived peroxide with a p K a of 6. It also yields secondary free radical intermediates such as nitrogen dioxide and carbonate radicals. Much of nitric oxide- and superoxide-dependent cytotoxicity resides on peroxynitrite, which affects mitochondrial function and triggers cell death via oxidation and nitration reactions. Peroxynitrite is an endogenous toxicant but is also a cytotoxic effector against invading pathogens. The biological chemistry of peroxynitrite is modulated by endogenous antioxidant mechanisms and neutralized by synthetic compounds with peroxynitrite-scavenging capacity. Free radicals typically react fast with each other via radical-radical coupling reactions.
Art modeling studio cherish
Hidden categories: Articles with short description Short description is different from Wikidata Chemical pages without ChemSpiderID Articles containing unverified chemical infoboxes Chembox image size set. This class of metal-based drugs, initially conceived as SOD mimics 87 , readily reacts with peroxynitrite 13 and has been used to attenuate peroxynitrite-dependent cytotoxicity Indeed, radical combination reactions usually occur at near diffusion-controlled rates 1. Zhu L. Equation 3. Alternative approaches have been also used, with the application of competition kinetics with reference reactions of known rate constants Acknowledgments I thank Drs. Then, MnP protect mitochondria from peroxynitrite-mediated toxicity both in vitro and in vivo. The relevance of the intramitochondrial formation of peroxynitrite is underscored by the established observation of mitochondrial protein nitration in pathological states and even under basal conditions reviewed in Ref. The remarkable velocity for peroxiredoxin reactions with peroxynitrite Table 1 extended earlier observations for H 2 O 2 30 ; the molecular determinants of such reactivity are under scrutiny but seem to depend of the stabilization of the enzyme-activated complex It can account for enzyme inactivation This process is largely observed in vivo under inflammatory conditions.
In this review we provide an analysis of the biochemistry of peroxynitrite and tyrosine nitration. In addition, peroxynitrite anion can secondarily evolve to secondary radicals either via its fast reaction with CO 2 or through proton-catalyzed homolysis. Peroxynitrite can cause protein tyrosine nitration in vitro and in vivo.
It also yields secondary free radical intermediates such as nitrogen dioxide and carbonate radicals. Uric acid is also a physiological substrate of myeloperoxidase 98 and may therefore interfere in heme peroxidase-dependent nitration reactions as well. Bringold U. Heart Circ. Chemical compound. Moreover, the recent characterization of boronate-based probes that react quickly with peroxynitrite 95 Table 1 provides possibilities for a more specific detection and even quantitation of peroxynitrite by bioanalytical and bioimaging techniques. Cytotoxic Effector against Invading Pathogens The cytotoxic properties of peroxynitrite can be also utilized by immune system cells to combat infecting microorganisms. How the radicals do the job. Peroxynitrite-mediated Protein Tyrosine Nitration Peroxynitrite does not react directly with tyrosine Similarly, NX indicated an uncharacterized nitrogen-containing product, later proved to be nitrite. Yamakura F. Open in a separate window. Methods Enzymol. Buettner G.

You are mistaken. I suggest it to discuss. Write to me in PM, we will communicate.
It is remarkable, it is very valuable phrase