Pf5 lewis structure
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? American National Curriculum.
What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule? Therefore, there are eight valence electrons in total. The Lewis structure shows that nitrogen has one lone pair and is bonded to three fluorine atoms. To explain the stability of NF3, we need to
Pf5 lewis structure
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1]. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism , by which the axial and equatorial fluorine atoms rapidly exchange positions. The apparent equivalency of the F centers in PF 5 was first noted by Gutowsky. Stephen Berry , after whom the Berry mechanism is named. Electron diffraction and X-ray crystallography do not detect this effect as the solid state structures are, relative to a molecule in solution, static and can not undergo the necessary changes in atomic position. Phosphorus pentafluoride is a Lewis acid. This property is relevant to its ready hydrolysis. A well studied adduct is PF 5 with pyridine. With primary and secondary amines, the adducts convert readily to dimeric amido-bridged derivatives with the formula [PF 4 NR 2 ] 2.
No Try it.
Submitted by Patricia S. We will assign your question to a Numerade educator to answer. Draw the Lewis structure for the phosphorus pentafluoride PF5 molecule. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure for phosphorus pentachloride, please include the following.
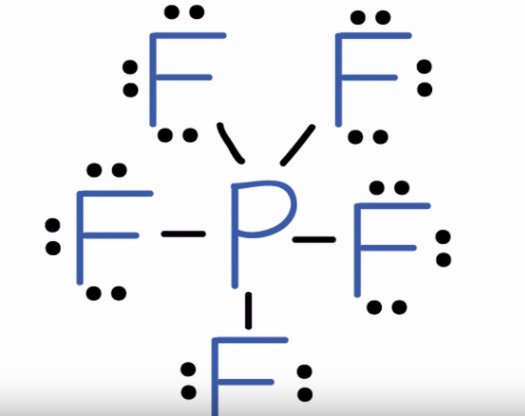
Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms. This molecule is also known as the halide gas as it consists of Fluorine a halogen atom. To understand the physical and chemical properties of this molecule, it is essential to know its Lewis Structure. This structure helps to understand the arrangement of atoms, bond formation and shape of the molecule. However, for knowing the Lewis Structure of any compound, one first needs to know the total number of valence electrons. Phosphorus has 5 valence electrons in its outer shell. Fluorine has 7 valence electrons in its outer shell, but as there are 5 fluorine atoms, we will multiply the number by 5.
Pf5 lewis structure
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1]. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :.
1.69 m height in feet
With primary and secondary amines, the adducts convert readily to dimeric amido-bridged derivatives with the formula [PF 4 NR 2 ] 2. Try it in the Numerade app? Next Previous. Was the language and grammar an issue? Question 4. The treated polymer was found to contain 4. We'll put the Phosphorus in the center, and then the Fluorines, we have five of them, let's put them around it like this. Five plus 40 valence electrons. DyF 3. Consider the molecules PF3 and PF5. That means we have 30 left.
Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Expert Solution. Q: onsider these reactions, where M represents a generic metal. A: Some chemical reactions are given and we need to predict the indicated chemical is whether Lewis…. Help us make our solutions better Rate this solution on a scale of star. Ball, Edward Mercer. Q: You have mL of a buffer solution conatining 0. A: Calcium hydroxide reacts with hydrochloric acid to form calcium chloride and water. Identify each compound as molecular,…. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Q: Match each compound to its role in the Tetraphenylcyclopentadienone reaction First paragraph only,…. Handbook of Preparative Inorganic Chemistry. Step 6: Next, we determine the central atom by identifying the atom with the lowest electronegativity. Other anions.

I am sorry, that I interrupt you, I too would like to express the opinion.
This variant does not approach me. Perhaps there are still variants?
It is remarkable, it is very valuable answer