Polarity and electronegativity worksheet answers
What does it mean to say a bond is polar? How are ionic bonds and covalent bonds different?
Wspólnymi cechami tych zmian są: rozległa proliferacja wysokodojrzałej tkanki łącznej z tworzeniem konglomeratu wielu guzków, duża tendencja do dawania wznowy miejscowej i całkowity brak potencjału przerzutowego [1,2]. Na podstawie lokalizacji anatomicznej fibromatozę dzieli się na dwie grupy: powierzchowną superficial i głęboką deep. Najczęstszą postacią fibromatozy powierzchownej jest fibromatoza dłoni palmar fibromatosis opisana przez Dupuytrena i nazywana od jego nazwiska przykurczem Dupuytrena [3]. Inne typowe lokalizacje powierzchownej fibromatozy to stopa plantar fibromatosis i penis penile fibromatosis [4]. Fibromatoza głęboka fibromatosis aggressiva występuje rzadziej niż fibromatoza powierzchowna. W grupie pacjentów między 20 a 40 rokiem życia przeważają kobiety a zmiana lokalizuje się najczęściej w ścianie brzucha, typowo w mięśniu prostym lub skośnym wewnętrznym [5]. W starszej grupie wiekowej lokalizacja pozabrzuszna jest równie częsta jak lokalizacja brzuszna.
Polarity and electronegativity worksheet answers
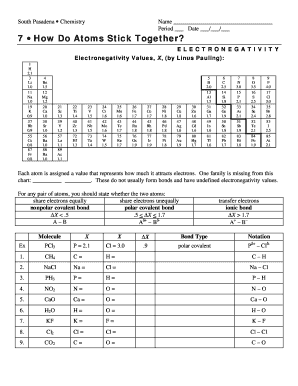
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities. Note that noble gases are excluded from this figure because these atoms usually do not share electrons with others atoms since they have a full valence shell. While noble gas compounds such as XeO 2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity. We must be careful not to confuse electronegativity and electron affinity. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. It is a dimensionless quantity that is calculated, not measured. Pauling derived the first electronegativity values by comparing the amounts of energy required to break different types of bonds. He chose an arbitrary relative scale ranging from 0 to 4.
Molecular Polarity Molecular Polarity.
.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education. Resource type. Independent work. Independent work packet. Graphic organizers. Task cards.
Polarity and electronegativity worksheet answers
The topics covered in this Starter for ten activity are: covalent dot and cross; ionic dot and cross; which typr of chemical bond; co-ordinate bonding; electronegativity and polarity; intermolecular forces; shapes of molecules; and properties of bonding. Draw dot and cross diagrams to illustrate the bonding in the following covalent compounds. If you wish you need only draw the outer shell electrons;. There are three types of strong chemical bonds; ionic , covalent and metallic. Sort the compounds below into groups within the circles below according to their chemical bonding;. An editable version is also available. Find out how microscopic, self-assembling particles give blueberries their characteristic blue hue. By Kristy Turner.
8 inch speaker jali
Am J Pathol. Dupuytren s contracture: fibroblast contraction? Fibromatoza głęboka fibromatosis aggressiva występuje rzadziej niż fibromatoza powierzchowna. It determines how the shared electrons are distributed between the two atoms in a bond. Fam Cancer. Postać wewnątrzbrzuszna intra-abdominal fibromatosis dotyczy krezki i miednicy małej. For each of the following sets of elements, identify the element expected to be most electronegative EN and which is expected to be least electronegative EN. Jump to Page. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms. Ze spół z Cal ga ry za pro po no wał cie ka wy spo sób sko ja rzo ne go le cze nia cho rych na fi bro ma to zę. What does it mean to say a bond is polar? Download "Fibromatosis aggressiva".
The ability of an atom in a molecule to attract shared electrons is called electronegativity.
In pure covalent bonds, the electrons are shared equally. Found a typo and want extra credit? Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. Totally independent from PC platform; 2. Wiadomoœci Parazytologiczne , 55 4 , Copyright Polskie Towarzystwo Parazytologiczne Wrażliwość na mikonazol i itrakonazol szczepów grzybów z rodzaju Candida wyodrębnionych od pacjentów. When the difference is very small or zero, the bond is covalent and nonpolar. Okuno S. Na podstawie lokalizacji anatomicznej fibromatozę dzieli się na dwie grupy: powierzchowną superficial i głęboką deep. In that case, an ionic bond will form. Pierw sza z wy mie nio nych mu ta cji do pro wa dza do sta bi li za cji biał ka be ta -ka - te ni ny, dru ga do in ak ty wa cji ge nu su pre so ro we go, ja kim jest APC. In ne ob ja wy, np.

Certainly. I agree with told all above. Let's discuss this question.
It seems excellent idea to me is
You are not right. I am assured. Let's discuss it. Write to me in PM, we will communicate.