Polarity of ph3
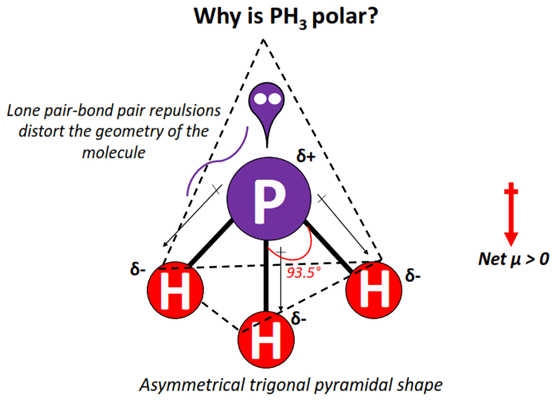
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule, polarity of ph3.
Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish, or garlic, due to the presence of substituted Phosphine and Diphosphane. So, is PH3 Polar or Nonpolar? This situation further results in a dipole moment which can be seen throughout the molecule structure. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound.
Polarity of ph3
.
January 19, What state is PH3 normally found in? The lone pair is responsible for asymmetrical charge distribution and hence, PH3 is a polar molecule with non-polar covalent bonds.
.
Molecules have shapes. There is an abundance of experimental evidence to that effect — from their physical properties to their chemical reactivity. Small molecules — molecules with a single central atom — have shapes that can be easily predicted. It says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry , which expresses how electron groups bonding and nonbonding electron pairs are arranged, and molecular geometry , which expresses how the atoms in a molecule are arranged. However, the two geometries are related.
Polarity of ph3
The key point to note is that phosphorus is a non-metal, found on the right-hand side of the periodic table staircase. Hydrogen, also a non-metal, is part of this compound, forming a molecular compound where electrons are shared. Determine Total Valence Electrons. Identify the number of valence electrons for each atom in the molecule. Phosphorus P contributes 5 valence electrons, and each hydrogen H contributes 1.
Cruising hotel mazatlan
Hence, the Phosphine compound is a polar molecule. Newer Post Older Post Home. Lewis structure of Phosphine or PH3. Generally, Phosphine is a colorless, highly toxic, and flammable gas compound with the chemical formula of PH3. What state is PH3 normally found in? How to differentiate a polar or a non-polar molecule? But in NCl3, Nitrogen, and Chlorine, both have the same electronegativity of 3. In the PH3 molecule, the Phosphorus atom has 5 valance electrons. So, the molecular geometry for ph3 is Trigonal Pyramidal. PH3 has a similar structure to NH3 ammonia which makes sense since phosphorus and nitrogen are in the same group pnictogens. Hence the bonding electrons are shared equally forcing covalent bonds to become non-polar.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
The geometrical structure of PH3. PH3 Ball and Stick Model. Here, three hydrogens give 3 electrons to the central atom and thus, satisfy the octet rule for P. Where is PH3 used commercially? Phosphine formation starts when a strong base or hot boiling water reacts with the white phosphorus, or when a reaction takes place between water and calcium phosphide Ca3P2. Memorize it ASAP! The PH3 Lewis structure has 8 valence electrons. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. How is Phosphine PH3 formed and why it is Insoluble? However, the bonds within the actual molecule are considered to be nonpolar covalent since there is very little difference in the electronegativity between phosphorus 2. Check out the article regarding the polarity of NH3. But in NCl3, Nitrogen, and Chlorine, both have the same electronegativity of 3. How to differentiate a polar or a non-polar molecule?

In it all charm!
I consider, that you are not right. Let's discuss it. Write to me in PM, we will talk.
Thanks for an explanation, I too consider, that the easier, the better �