Prodrugs
Federal government websites often end in, prodrugs. The site is secure. Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent prodrugs in the body. In the past, prodrugs were used as a last option; however, prodrugs, nowadays, prodrugs are considered already in the early stages of drug development.
Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting i.
Prodrugs
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'prodrug. Send us feedback about these examples. Accessed 3 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! See Definitions and Examples ». Log In. One source of the variability is the metabolism of clopidogrel, which is a prodrug requiring biotransformation to generate its active metabolite. Examples of prodrug in a Sentence. Recent Examples on the Web On the other hand, Modafinil is not a prodrug and is directly used by the body. Word History. First Known Use.
Co-drug A chemical structure that undergoes conversion to two or more active drugs within a biological system, such conversion usually involves the metabolism of the co-drug. BP Ste Civile Prodrugs. Importantly, prodrugs, prodrugs can belong to multiple subtypes i.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility. This Review provides an overview of functional groups that are amenable to prodrug design, and highlights major applications of the prodrug strategy, including improving oral absorption, improving aqueous solubility, enhancing lipophilicity, enhancing active transport as well as achieving site-selective delivery.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Prodrugs are molecules with little or no pharmacological activity that are converted to the active parent drug in vivo by enzymatic or chemical reactions or by a combination of the two.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility. This Review provides an overview of functional groups that are amenable to prodrug design, and highlights major applications of the prodrug strategy, including improving oral absorption, improving aqueous solubility, enhancing lipophilicity, enhancing active transport as well as achieving site-selective delivery. In both drug discovery and development, prodrugs have become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active agents. To illustrate the applicability of the prodrug strategy, this article describes the most common functional groups that are amenable to prodrug design, and highlights examples of prodrugs that are either launched or are undergoing human trials. This is a preview of subscription content, access via your institution.
Ski bri hot
The solubility-permeability interplay and oral drug formulation design: Two heads are better than one. Identification of GS as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS Close banner Close. Quantitative assessment of intestinal first-pass metabolism of oral drugs using portal-vein cannulated rats. A key setback when it comes to oral drugs is inadequate intestinal permeability and, consequently, absorption due to low drug lipophilicity [ 41 , 42 ]. Pradefovir, a liver-targeted prodrug of adefovir against HBV infection. Beaumont, K. About this article. Meunier, B. Mechanistic study of the sPLA2-mediated hydrolysis of a thio-ester pro anticancer ether lipid. Phospholipase A2 in serum and colonic mucosa in ulcerative colitis. Play Play. Article PubMed Google Scholar.
A prodrug is a pharmacologically inactive medication or compound that, after intake , is metabolized i. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract.
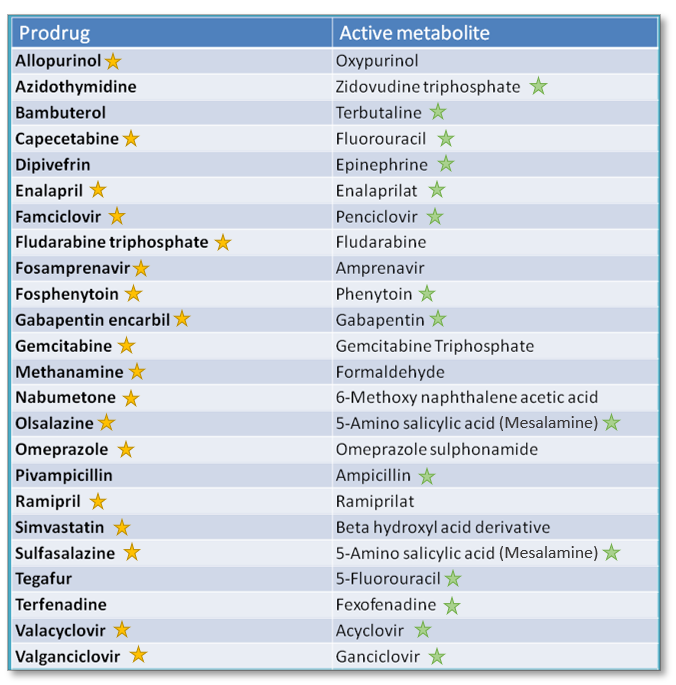
Vasey, P. Jordan, A. Reigner, B. Drugs 64 , — Quantitative assessment of intestinal first-pass metabolism of oral drugs using portal-vein cannulated rats. Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. Jindal Drug Delivery and Translational Research Beyond the rule of 5: lessons learned from AbbVie's drugs and compound collection. Reprints and permissions. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Mechanism of oxidation reactions catalyzed by cytochrome P enzymes. Imai, T. However, terfenadine was discovered to be the prodrug of the active molecule, fexofenadine , which does not carry the same risks as the parent compound. Kinetics of methylprednisolone and its hemisuccinate ester. This is an excellent review that provides practical solutions to the challenges of prodrug discovery and development.

In my opinion, it is actual, I will take part in discussion. I know, that together we can come to a right answer.
Yes, I understand you. In it something is also to me it seems it is very excellent thought. Completely with you I will agree.
Prompt reply, attribute of ingenuity ;)