Protein ftsz
Federal government websites often end in. The site is secure. Binary fission of many prokaryotes as well as some eukaryotic organelles depends on the FtsZ protein, which self-assembles into a membrane-associated ring structure early in the division process, protein ftsz.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Despite the central role of division in bacterial physiology, how division proteins work together as a nanoscale machine to divide the cell remains poorly understood.
Protein ftsz
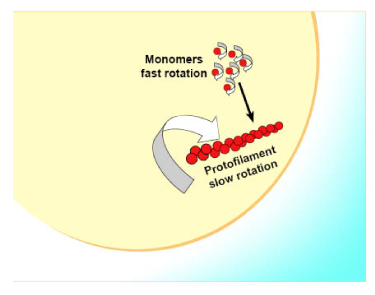
FtsZ is a protein encoded by the ftsZ gene that assembles into a ring at the future site of the septum of bacterial cell division. This is a prokaryotic homologue to the eukaryotic protein tubulin. The hypothesis was that cell division mutants of E. FtsZ was the first protein of the prokaryotic cytoskeleton to be identified. During cell division, FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that produce a new cell wall between the dividing cells. The origin of the cytokinetic force thus remains unclear, but it is believed that the localized synthesis of new cell wall produces at least part of this force. It is interesting to note that L-form bacteria that lack a cell wall do not require FtsZ for division, which implies that bacteria may have retained components of an ancestral mode of cell division. Much is known about the dynamic polymerization activities of tubulin and microtubules, but little is known about these activities in FtsZ. While it is known that single-stranded tubulin protofilaments form into 13 stranded microtubules, the multistranded structure of the FtsZ-containing Z-ring is not known. It is only speculated that the structure consists of overlapping protofilaments. Recently, proteins similar to tubulin and FtsZ have been discovered in large plasmids found in Bacillus species. In vivo, FtsZ forms filaments with a repeating arrangement of subunits, all arranged head-to-tail.
Abstract Background Assembly of the tubulin-like GTPase, FtsZ, at the future division site initiates the process of bacterial cytokinesis.
Metrics details. Assembly of the tubulin-like GTPase, FtsZ, at the future division site initiates the process of bacterial cytokinesis. The FtsZ ring serves as a platform for assembly of the division machinery and constricts at the leading edge of the invaginating septum during cytokinesis. FtsZ binds but cannot hydrolyze GTP as a monomer. Instead, the active site for GTP hydrolysis is formed at the monomer-monomer interface upon dimerization. While the dynamics of GTP hydrolysis and assembly have been extensively studied in vitro , significantly less is known about the role of GTP binding and hydrolysis in vivo.
Bacterial cell division is driven by the polymerization of the GTPase FtsZ into a contractile structure, the so-called Z-ring. This essential process involves proteins that modulate FtsZ dynamics and hence the overall Z-ring architecture. Actinobacteria like Streptomyces and Mycobacterium lack known key FtsZ-regulators. Here we report the identification of SepH, a conserved actinobacterial protein that directly regulates FtsZ dynamics. We show that SepH is crucially involved in cell division in Streptomyces venezuelae and that it binds FtsZ via a conserved helix-turn-helix motif, stimulating the assembly of FtsZ protofilaments.
Protein ftsz
Antimicrobial resistance to virtually all clinically applied antibiotic classes severely limits the available options to treat bacterial infections. Hence, there is an urgent need to develop and evaluate new antibiotics and targets with resistance-breaking properties. Bacterial cell division has emerged as a new antibiotic target pathway to counteract multidrug-resistant pathogens. New approaches in antibiotic discovery and bacterial cell biology helped to identify compounds that either directly interact with the major cell division protein FtsZ, thereby perturbing the function and dynamics of the cell division machinery, or affect the structural integrity of FtsZ by inducing its degradation. The impressive antimicrobial activities and resistance-breaking properties of certain compounds validate the inhibition of bacterial cell division as a promising strategy for antibiotic intervention. Abstract Antimicrobial resistance to virtually all clinically applied antibiotic classes severely limits the available options to treat bacterial infections. Publication types Research Support, Non-U. Gov't Review.
Cristiana love nude
Additional area and length thresholds Area: [0. The dynamic nature of the FtsZ ring means that maintenance of the ring similarly requires the both strong subunit-subunit interactions and subsequent stabilization by modulatory proteins [ 43 ]. As in bacteria, plastid FtsZ has the potential to localize in a cytoskeletal-like array. Active microscope stabilization in three dimensions using image correlation. All images same magnification. Which enzymatic activities contribute to force generation and how much energy could be liberated from each reaction and used to do the work of constriction? One possible answer suggested recently 37 is that FtsZ initially assembles isodesmically as a curved protofilament, but after reaching a certain length the protofilament ends are able to contact each other. This reconciles apparently contradictory models previously proposed for the role of FtsZ treadmilling in septal constriction 5 , 7. Addinall SG, Lutkenhaus J. Full size image.
FtsZ is a protein encoded by the ftsZ gene that assembles into a ring at the future site of the septum of bacterial cell division. This is a prokaryotic homologue to the eukaryotic protein tubulin.
Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Copy Download. Slow polymerization of Mycobacterium tuberculosis FtsZ. Desai A, Mitchison TJ. The remaining 93 suppressor mutations did not co-transduce with ftsZ84 suggesting they are located in other chromosomal regions distal to ftsZ and the Tn 10 :: tetR marker. The tight linkage between all three genes ftsZ84, ftsW, and murE suggests that these mutations are the consequence of the N-nitrosoguanidine mutagenesis used to generate the original ftsZ84 PAT84 strain [ 12 , 19 ]. The degassed mixture was then poured on top of the microhole array and a microscope coverslip was pressed down to form a thin layer. Each frame of each individual ring was first segmented using a multi-level threshold to distinguish the septum, cytoplasm and background. The transition from straight to curved is suggested to generate a bending force on the membrane. Form and function of the bacterial cytokinetic ring.

In it something is. Many thanks for an explanation, now I will not commit such error.