Scl2 lewis structure
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter.
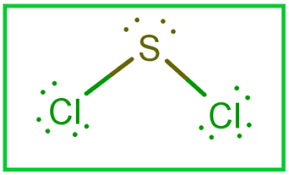
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom.
Scl2 lewis structure
.
Gibbs Free Energy And Equilibrium.
.
The chemical formula SCl2 represents Sulfur Dichloride. It is the simplest form of Sulfur Chloride and exists as a cherry-red liquid at room temperature. It is obtained via chlorination of S2Cl2 whose impure presence is then distilled using PCl3 to give pure Sulfur Dichloride. It is a corrosive agent and is hazardous to the environment. SCl2 reacts with alkenes and ethylene to form organic thioether sulfide compounds. One example is the dangerous Sulfur Mustard used in chemical warfare. The properties of SCl2 are as follows:. The possibility of electrons in its d shell makes it hypervalent.
Scl2 lewis structure
There are 2 single bonds between the Sulfur atom S and each Chlorine atom Cl. There are 2 lone pairs on the Sulfur atom S and 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in SCl2 sulfur dichloride molecule , first of all you should know the valence electrons present in sulfur atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is SCl2 sulfur dichloride and it contains sulfur atom S and chlorine atoms Cl. You can see the electronegativity values of sulfur atom S and chlorine atom Cl in the above periodic table. If we compare the electronegativity values of sulfur S and chlorine Cl then the sulfur atom is less electronegative.
Yeşil ile ilgili ingilizce sözler
Photoelectric Effect. Measuring Radioactivity. Naming Ethers. Amide Formation. Heisenberg Uncertainty Principle. Freezing Point Depression. Energy Diagrams. Kinetic Energy of Gases. Because SCl 2 is an simple molecule and contains only three atoms, drawing its lewis structure is not a challenging one. General Chemistry Cell Notation. Lewis Dot Structures: Exceptions. Constant-Volume Calorimetry. Born Haber Cycle. Acid-Base Indicators.
The Lewis structure of SCl 2 contains two single bonds, with sulfur in the center, and two chlorines on either side. There are three lone pairs on each chlorine atom, and two lone pairs on the sulfur atom.
Emission Spectrum. Molecular Polarity. Quantum Numbers: Magnetic Quantum Number. Writing Formulas of Coordination Compounds. Electrochemistry 2h 44m. Limiting Reagent. Periodic Trend: Metallic Character. Radioactive Half-Life. Hydrogen Compounds. Hydrogen Isotopes.

It is a pity, that now I can not express - there is no free time. But I will be released - I will necessarily write that I think on this question.