Sf2 hybridization
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2its molecular geometry and shape, sf2 hybridization. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total sf2 hybridization electrons for Sulphur Difluoride.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details.
Sf2 hybridization
.
Hence, the charge on the compound is not evenly distributed thus we can be sure that the dipole moment is not going to be zero. Sf2 hybridization Structure is nothing but an arrangement of valence electrons between different atoms.
.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. So, Sulphur Difluoride has a total of 20 valence electrons. Lewis Structure is the pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. And now that we know the total valence electrons of SF2, we will start making the Lewis Dot Structure for this molecule. Firstly, place the Sulphur atom in the centre as it is less electronegative than Fluorine. So it will be in the central position with both these Fluorine atoms on the terminal ends.
Sf2 hybridization
Sulfur difluoride SF2 has the composition of one sulfur and two fluorine atoms. What is the molecular geometry of sulfur difluoride?. Drawing and predicting the SF2 molecular geometry is very easy by following the given method.
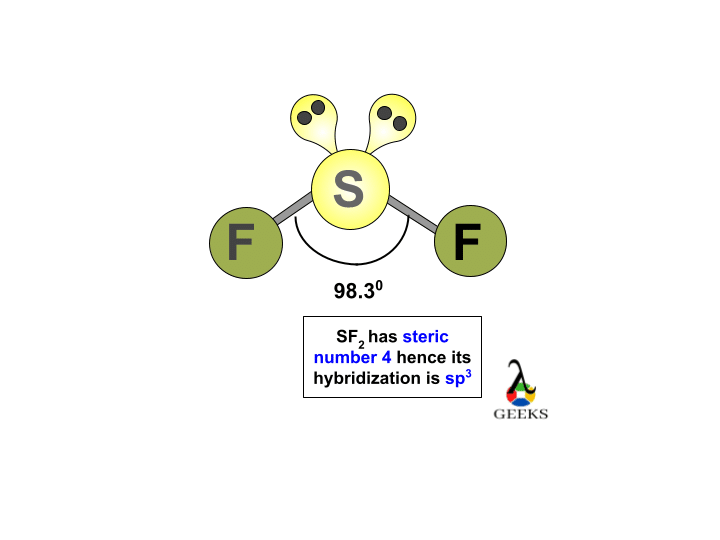
3 storey modern house design
So both the Fluorine atoms form a single bond with the Sulphur atom by sharing one valence electron of the Sulphur atom. And so on…. The molecules with linear geometry have bond angles of degrees but here as the shape of the molecule is bent due to the lone pairs on the Sulphur atom, both Fluorine atoms are pushed downwards, deviating the bond angle of F-S-F from to 98 degrees. Due to which there are repulsions and these repulsive forces lead to bent geometry. And there are three lone pairs of electrons on each Fluorine atom, which makes it a total of six pairs of lone electrons for the SF2 compound as a whole. This is because it is less electronegative than the other atom of the compound which is Fluorine. The repulsion between the atoms and their corresponding electrons should be minimum and to achieve that a compound takes a unique shape. One pair of electron sharing means that only a single bond is formed between Sulfur and two atoms of Fluorine each. And due to this vast difference in electronegativity, there will be a dipole moment between Sulphur and Fluorine atoms. Similarly, the electronic configuration of Fluorine is 1s2 2s2 2p5. According to VSEPR theory, each atom in a compound is arranged in a way that the compound becomes stable in nature. Here, the November 28, We hope that the article was insightful enough for you and that you got a good grasp of the discussed topic.
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o.
Contents show. And, the valence electrons of Fluorine are 7 in number. In the case of SF2, you can look at the below-given diagram to understand how the electrons are placed in different energy orbitals. And there are three lone pairs of electrons on each Fluorine atom, which makes it a total of six pairs of lone electrons for the SF2 compound as a whole. The repulsion between the atoms and their corresponding electrons should be minimum and to achieve that a compound takes a unique shape. In fact, both Fluorine atoms are also sp3 hybridized. Thus, the molecular geometry is of the type AX2E2 which leads us to the conclusion that the compound is non-linear or is bent. Fluorine atoms need one valence electron to complete its octet so it will share one valence electron of the Sulphur atom. Here is a MO diagram of another bent compound ie; bent shaped SO2 molecule and how the energies are distributed. Related Posts.

0 thoughts on “Sf2 hybridization”