Shape of becl2 according to vsepr theory
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
Submitted by Marilyn R. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What would be its associated molecular geometry?
Shape of becl2 according to vsepr theory
The central atom has no lone pair and there are two bond pairs. Hence, it has a linear shape. The central atom has no lone pair and there are three bond pairs. Hence, it is of the type A B 3. Hence, it is trigonal planar. The central atom has no lone pair and there are four bond pairs. Hence, the shape of SiCl4 is tetrahedral being the A B 4 type molecule. The central atom has no lone pair and there are five bond pairs. Therefore, the shape is trigonal bipyramidal. The central atom has one lone pair and there are two bond pairs. The shape is Bent. The central atom has one lone pair and there are three bond pairs. Therefore, the shape is trigonal pyramidal.
The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is.
The shapes of the molecules is determined mainly by the electrons surrounding the central atom. In a molecule EX n , the valence shell electron pair around the central atom E and the E-X single bonds are very important due to the repulsion in which determine the shape of the molecule. The repulsions decrease in order of: lone pair-lone pair, lone pair-bonding pair, bonding pair-bonding pair. At the same time, the repulsion would decrease in order of: triple bond-single bond, double bond-single bond, and single bond-single bond if the central atom E has multiple bonds. The difference between the electronegativities of E and X also determine the repulsive force between the bonding pairs. If electron-electron repulsive force is less, then more electron density is drawn away from the central atom E.
The central atom has no lone pair and there are two bond pairs. Hence, it has a linear shape. The central atom has no lone pair and there are three bond pairs. Hence, it is of the type A B 3. Hence, it is trigonal planar. The central atom has no lone pair and there are four bond pairs. Hence, the shape of SiCl4 is tetrahedral being the A B 4 type molecule. The central atom has no lone pair and there are five bond pairs. Therefore, the shape is trigonal bipyramidal. The central atom has one lone pair and there are two bond pairs.
Shape of becl2 according to vsepr theory
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom.
Depeche mode support act twickenham 2023
Thus BeH 2 is designated as AX 2. With the sulfur IV fluoride SF 4 molecule, electron pairs are based on a trigonal bipyramid geometry. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. The Shapes of Molecules and Ions and bond angles related to their Electronic Structure - mainly inorganic molecules on this page. Draw the Lewis structure for NO2-, then answer the following questions. Therefore, the shape of the molecules are arranged so that the energy is minimized. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Hence, it is trigonal planar. ClF 5. Cl-Be-Cl linear shape. There are five groups around sulfur, four bonding pairs and one lone pair. Get Better Grades Now. The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. PH3: This molecule has three atoms bonded to the central P atom, with one lone pair.
Drawing and predicting the BeCl2 molecular geometry is very easy.
Individual bond dipole moments are indicated in red. Download Important Formulas pdf. The central atom, boron, contributes three valence electrons, and each chlorine atom contributes seven valence electrons. BeF2 BeF2 is another molecule type that constitutes two places in a valence shell. Millions of real past notes, study guides, and exams matched directly to your classes. What is the shape of PF 5? Diatomic molecules. Tin has one lone pair. What is the shape of the HgCl2 molecule and its bond angle? Already have an account? From this we can describe the molecular geometry.

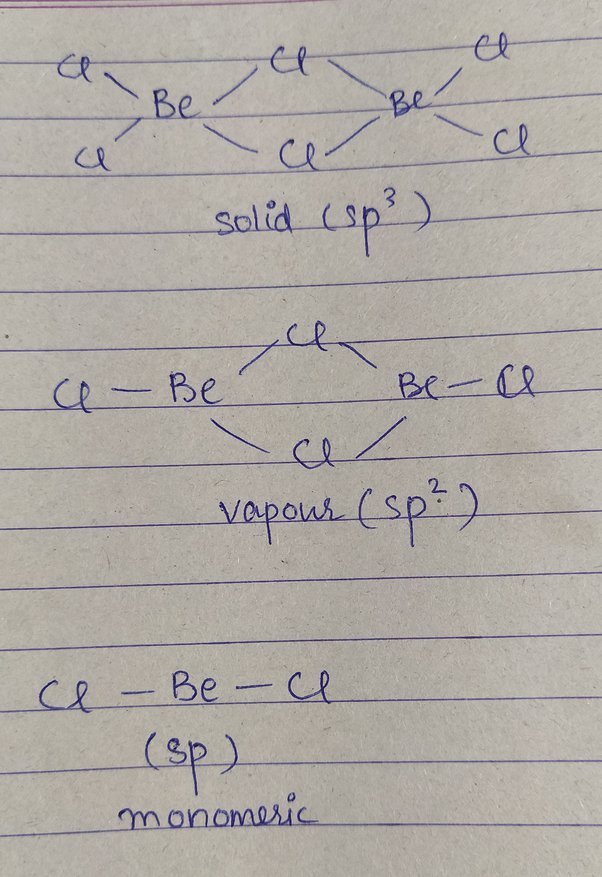
Yes, really. I join told all above. Let's discuss this question.