Shape of sf4 according to vsepr theory
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however.
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shell electron-pair repulsion VSEPR theory. The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules. There are only two places in the valence shell of the central atom in BeF 2 where electrons can be found. Repulsion between these pairs of electrons can be minimized by arranging them so that they point in opposite directions.
Shape of sf4 according to vsepr theory
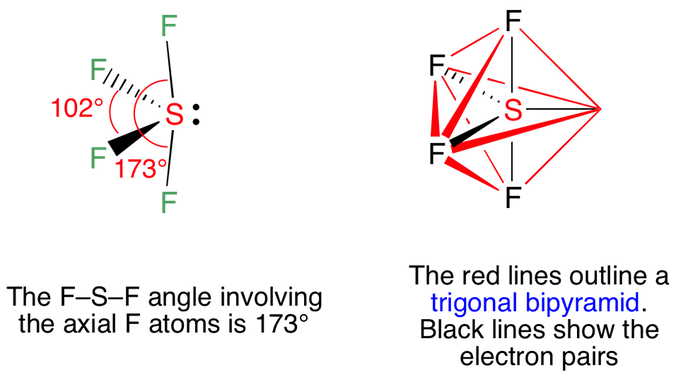
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry. Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see not bonded. So, that bends the axial fluorines together a bit. What is the shape of SF4 including bond angles? Truong-Son N.
Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP.
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model.
Shape of sf4 according to vsepr theory
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Sulfur and fluorine will combine to form four S-F single bonds. Sulfur will use four valence electrons to bond with the four fluorine atoms. Hence, it will have one lone pair of electrons, while each fluorine atom will have six []. Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two.
Young and restless tucker mccall
As with SO 2 , this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. The central atom, carbon, contributes four valence electrons, and each oxygen atom contributes six. This can be described as a trigonal bipyramid with three equatorial vertices missing. Consequently, molecules with these geometries always have a nonzero dipole moment. Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see not bonded. We expect the concentration of negative charge to be on the oxygen, the more electronegative atom, and positive charge on the two hydrogens. This charge polarization allows H 2 O to hydrogen-bond to other polarized or charged species, including other water molecules. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. With 18 valence electrons, the Lewis electron structure is shown below. BeF 2 and BF 3 are both two-dimensional molecules, in which the atoms lie in the same plane. This designation has a total of four electron pairs, three X and one E. But the results of the VSEPR theory can be used to predict the positions of the nuclei in these molecules, which can be tested experimentally. AX 2 Molecules: CO 2 1. Consequently, the bond dipole moments cannot cancel one another, and the molecule has a dipole moment. For example, in a molecule such as CH 2 O AX 3 , whose structure is shown below, the double bond repels the single bonds more strongly than the single bonds repel each other.
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds.
One carbon bonded to nitrogen and another carbon double bonded to the nitrogen. Looking at this, we can say that the number of regions of electron density is 5. As with SO 2 , this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. We expect the LP—BP interactions to cause the bonding pair angles to deviate significantly from the angles of a perfect tetrahedron. Although there are lone pairs of electrons, with four bonding electron pairs in the equatorial plane and the lone pairs of electrons in the axial positions, all LP—BP repulsions are the same. But if the nonbonding electrons are placed in an equatorial position, they will be 90 o away from only two pairs of bonding electrons. Consequently, the bond dipole moments cannot cancel one another, and the molecule has a dipole moment. As you learned previously, the Lewis electron structure of one of three resonance forms is represented as. There are six nuclei, so the molecular geometry of SF 6 is octahedral. Sulfur has one lone pair. The VSEPR model can be used to predict the structure of somewhat more complex molecules with no single central atom by treating them as linked AX m E n fragments.

You have missed the most important.