So2 hybridization structure
Step 2: Formula used for calculation:. Step 3: Calculation for Hybridisation of SO 2 :. Sulfur dioxide dissolves in water to produce an acid.
The central atom, Sulfur, has 3 electron clouds in this molecule. The primary sulphur atom is bonded to two oxygen atoms. Sulfur in its ground state has first shells fully filled and 6 electrons within the outermost shell. There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital. Now, there are 4 unpaired electrons three unpaired electrons in three 3p orbitals and one unpaired electron in one 3-D orbital. As the electrons forming sigma bonds and the lone pair want to be on an equal energy level, hybridization takes place.
So2 hybridization structure
.
During the formation of Sulfur dioxide SO 2the central atom i. The hybridization of the two oxygen atoms is sp 2 as properly.
.
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect.
So2 hybridization structure
The chemical formula of sulfur dioxide is SO2. It consists of one sulfur atom and two oxygen atoms. When sulfur burns in the air, it reacts with oxygen to produce sulfur dioxide. Sulfur dioxide is a colorless gas with a pungent odor and is commonly used in the production of sulfuric acid. To draw the SO2 Lewis Structure, the valence electrons of the sulfur and oxygen atoms are considered, and the electrons are arranged to minimize their repulsion. In the SO2 Lewis Structure, the sulfur atom is placed in the center, and the two oxygen atoms are attached to it by a double bond. The sulfur atom has six valence electrons, while each oxygen atom has six valence electrons.
Kieron top boy
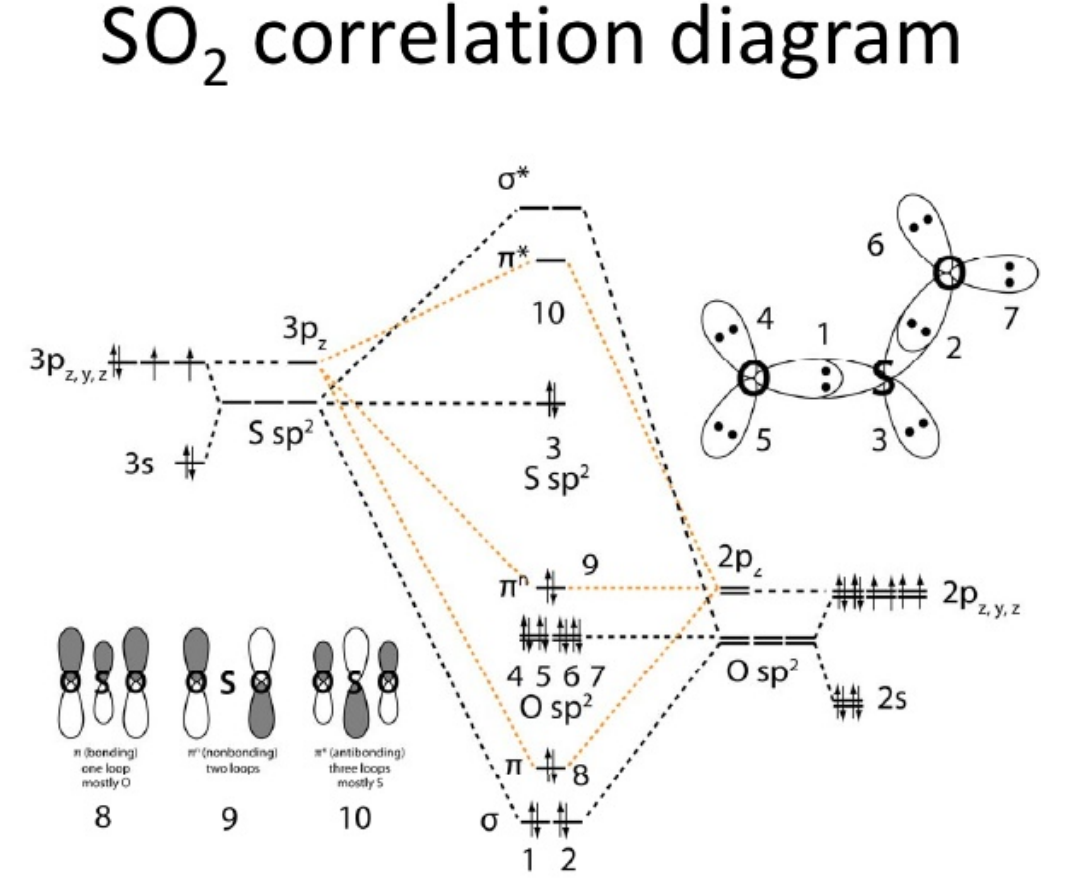
Byju's Answer. Sulfur desires 6 electrons, and so does oxygen. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital. During the formation of Sulfur dioxide SO 2 , the central atom i. Those account for the 2 lone pairs of electrons on every oxygen, and you may show by means of noticing how the MO diagram shows the black dashed-line contribution from only the sp 2 AOs of oxygen. Name one catalyst used industrially which speeds up the conversion of sulfur dioxide to sulfur tri-oxide. What is the formula mass for Sulfur dioxide? Then, sulfur dioxide is converted to sulfur trioxide which reacts with producing sulfuric acid. The antibonding MOs are empty. There are sp 2 orbitals of oxygen that don't overlap with the sp 2 orbitals of sulfur. That makes experience due to the fact that we had described the axis coming towards us as the z-axis, the 2pz and 3pz orbitals each lie alongside that axis, and we hadn't taken into consideration the ones orbitals but. This is orbital three and is categorized because the nonbonding "S sp 2 " MO within the middle of the diagram, below MO
Here, we will explain the hybridization of SO 2 in detail. Students will also learn how to determine the type of hybridization, and they will find information about SO 2 molecular geometry and bond angles.
The ultimate 3p and 3-D orbitals remain unhybridized. Why does this reaction supply energy? One 3s and 3p orbitals get hybridized to shape three same sp2 hybrid orbitals. What is the name of the compound formed between sulfur trioxide and sulfuric acid? What is the mass of 1 m o l e of sulfur dioxide? What is the hybridization of sulfur dioxide? The two unpaired electrons within the unhybridized orbitals participate in the formation of pi bonds. Consequently, the hybridization of the principal sulfur atom in this compound is sp 2. There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals. What is the physical state of the acid formed? Some bacteria obtain their energy by oxidizing sulfur, producing sulfuric acid as a by product, In the laboratory, or industrially, the first step in the conversion of sulfur to sulfuric acid is to produce sulfur dioxide.

It agree, it is an amusing phrase
In it something is. Now all became clear to me, Many thanks for the information.
What words... super, a brilliant idea