So4 lewis dot structure
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it. This satisfies the octet rule allowing nitrogen's valence shell of electrons to look just like the noble gas neon's.
So4 lewis dot structure
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures.
Third Law of Thermodynamics. Intro to Crystal Field Theory.
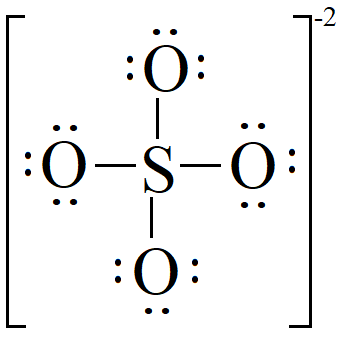
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule.
It is usually easier to figure out a problem if you can draw a picture, either mental or real, of what is happening. This is often done in physics and mathematics, and it is especially helpful when looking at the bonding, structure, physical properties, and reactivity of compounds. The most common picture, or model, of elements and compounds used is the Lewis Dot Structure. These pictures show you the type s of atom s involved, their position in the molecule, and where their valence electrons are situated. Dash each dash represents two electrons that are shared between two atoms as a covalent bond. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair; the other three pairs of electrons on each chlorine atom are called lone pairs.
So4 lewis dot structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table.
Gta 5 pc social club
Step 6. Periodic Table: Phases. The Ideal Gas Law: Density. De Broglie Wavelength. Physical Properties. Average Rate of Reaction. Intro to Hydrocarbons. Hence, the sulfur atom S is the center atom, and the oxygen atoms O are the outside atoms. Amphoteric Species. Radioactive Half-Life. In order to avoid confusion, we have to indicate in some manner where we get the other two electrons. Step 2.
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O.
Emission Spectrum. Calculate Oxidation Numbers. Extensive Properties. Periodic Trend: Electronegativity. Crystal Field Theory: Tetrahedral Complexes. Coordination Complexes. The Electron Configurations: Exceptions. Example SO 4 2- A rule of thumb is that the central atom atom which all the other atoms will be bound is the one furthest to the left or bottom in the periodic table. Quantum Numbers: Nodes. Intro to Chemical Kinetics. Step 4. Chemical Kinetics 0.

Between us speaking, in my opinion, it is obvious. I will not begin to speak on this theme.
Not in it an essence.