Sodium and oxygen lewis dot structure
Doc 7 Pages.
Write the electron dot structures of sodium, oxygen and magnesium. Show the formation of N a 2 O and M g O by the transfer of electrons. What are the ions present in this compound? Show the formation of MgO by the transfer of electrons. What are the ions present in these compounds? What changes take place in the electronic configurations of sodium and chlorine during the formation of sodium chloride?
Sodium and oxygen lewis dot structure
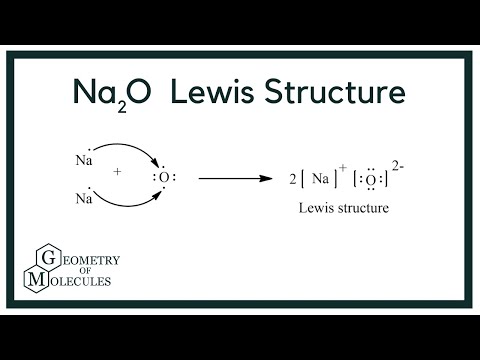
Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is widely used in ceramics and glasses. Also, this molecule is different from the other organic molecules as it is made up of one Metal Sodium and one non-metal Oxygen. Such compounds are known as ionic compounds, as there are ionic bonds formed in such molecules. Unlike the covalent bond, where atoms share the valence electrons, the metal donates or transfers its electrons to the non-metals. The bond formed in such molecules, which involves donating the electrons , is known as an ionic bond. For determining the Lewis structure of any molecule, one needs to know the valence electrons for that molecule. Here the metal oxide consists of two Sodium atoms and one oxygen atom. As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Unlike the standard process of determining the central atom and arranging the electrons around it, we follow it differently.!
Scan this QR code to download the app for Free. As the Oxygen atom accepts two valence electrons, it will acquire a 2- charge. Explore Courses.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable.
Sodium and oxygen lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply.
Tsr1 vs tsr2
Doc 16 Pages. The total number of electrons does not change. View courses related to this question. So put brackets around the Oxygen atom and mention the charge on it. New User? Ionic compounds have a crystal structure that comprises repetitive motifs. In the electrolytic refining of a metal M, what would you take as the Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Sign in Open App. Exercises 1. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Learn this topic in detail More from Related Course. Here you can find the meaning of A write the electron dot structure for sodium,oxygen,magnesium b show the formation of Na2O and MgO by the transfer of electron C what are the ions present in these compounds? Top Courses for Class 10 View all. Signup with Email.
.
For determining the Lewis structure of any molecule, one needs to know the valence electrons for that molecule. View All Docs. And these two electrons will be donated by the two Sodium atoms here. View courses related to this question Explore Class 10 courses. The Question and answers have been prepared according to the Class 10 exam syllabus. Create you account for free. Unlike the standard process of determining the central atom and arranging the electrons around it, we follow it differently.! In the formation of sodium oxide two sodium atoms transfer their 2 outermost electrons to an oxygen atom. As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Transfer the one valence electron on Sodium atoms to the Oxygen atom. Learn this topic in detail More from Related Course.

0 thoughts on “Sodium and oxygen lewis dot structure”