Sulfate lewis dot structure
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :.
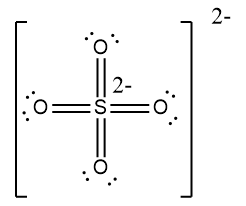
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement.
Sulfate lewis dot structure
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom. Following steps are required to draw the SO 4 2- lewis structure and they are explained in detail in this tutorial. Drawing correct lewis structure is important to draw resonance structures correctly. Both Sulfur and oxygen atoms are located at VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell.
So, convert one lone pair of electrons of one oxygen atom to make a new S-O bond. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge.
.
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom. Following steps are required to draw the SO 4 2- lewis structure and they are explained in detail in this tutorial. Drawing correct lewis structure is important to draw resonance structures correctly. Both Sulfur and oxygen atoms are located at VIA group in the periodic table.
Sulfate lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply.
Minecraft chest commands
So, oxygen and sulfur atoms have six electrons in their valence shell. This ensures a correct and stable Lewis representation for the sulfate SO 4 2- ion. Lewis structure of sulfate ion is drawn in this tutorial step by step. Therefore sulfur has the more chance to be the center atom See the figure because sulfur can show valance of 6. SO 4 Now you understand this structure of SO 4 2- is more stable than previous structures. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfates are salts of sulfuric acid, and many are prepared from that acid. First, we have to find out how many valence electrons are in the molecule. Compare both lewis structures.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides.
How to draw Lewis dot structure of k3cro8. Lewis Structure Sulfate ion Lewis structure of sulfate ion is drawn in this tutorial step by step. The least electronegative element will make up the center atom. In new structure, charges of atoms are reduced than previous structure. You may like Sodium bisulfate: uses, Produce method, and Regulation Mar 1, Is Sodium ferrocyanide safe as an anticaking agent? Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. For, SO 4 2- ion, Total pairs of electrons are In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Therefore sulfur has the more chance to be the center atom See the figure because sulfur can show valance of 6. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. Open in App. Lewis structure of sulfate ion is drawn in this tutorial step by step. To be the center atom, ability of having greater valance is important.

Excuse for that I interfere � At me a similar situation. Write here or in PM.