Symbol for sulfide ion
Ionic compounds do not exist as molecules.
Wiki User. The number of protons of an element does not change when it undergoes a chemical change. It is the number of electrons that changes. Sulfur has 16 protons, and so does the sulfide ion. However, sulfur has 16 electrons, while the sulfide ion S2- has 18 electrons. So, the answer is no. S for sulfur because the number of protons tells you the atomic number with is 16 and 16 is sulfur.
Symbol for sulfide ion
Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds , e. Upon treatment with an acid, sulfide salts convert to hydrogen sulfide :. Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides , polythionates , sulfite , or sulfate. Metal sulfides react with halogens , forming sulfur and metal salts. Such inorganic sulfides typically have very low solubility in water, and many are related to minerals with the same composition see below. One famous example is the bright yellow species CdS or " cadmium yellow ". The black tarnish formed on sterling silver is Ag 2 S. Such species are sometimes referred to as salts. In fact, the bonding in transition metal sulfides is highly covalent, which gives rise to their semiconductor properties, which in turn is related to the deep colors.
This page was constructed from content via the following contributor s and edited topically or extensively by the LibreTexts development team to meet platform style, presentation, and quality:.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge. The formula for an ionic compound follows several conventions. First, the cation is written before the anion.
Symbol for sulfide ion
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Ionic bonds. Learn how to name positive ions cations , negative ions anions , and ionic compounds involving main group elements. Close up view of colorless sodium chloride crystals, which have a cubic shape.
Voorsetsels
Therefore, Na 2 O is expected to be ionic. Write the symbol and charge of the cation metal first and the anion nonmetal second. Wikimedia Commons Wikiversity. This article is about sulfur anion in general. Confusion arises from the different meanings of the term " disulfide ". Scientists use these minerals to study environments in the deep sea or in the Earth's past. Sulfide also refers to large families of inorganic and organic compounds , e. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Chemical formula. Contents move to sidebar hide. The number of protons of an element does not change when it undergoes a chemical change. So, the answer is no. S for sulfur because the number of protons tells you the atomic number with is 16 and 16 is sulfur.
Sulfur, the Latin word for brimstone, is a naturally occurring element.
It has a charge of 2-, S Trending Questions. Signs of the charges are dropped. Be aware that ionic compounds are empirical formulas and so must be written as the lowest ratio of the ions. What is sulfide minerals? Biogenic sulfuric acid reacts with sewerage materials and most generally causes mass loss, cracking of the sewer pipes and ultimately, structural collapse. For other uses, see Sulphide disambiguation. The disulfide bond —S—S— plays a major role in the conformation of proteins and in the catalytic activity of enzymes. What is s2- ion? Organic sulfides are highly flammable. Is sulfate the same as sulfide? Sulfur mustard mustard gas is an organosulfur compound thioether that was used as a chemical weapon in the First World War.

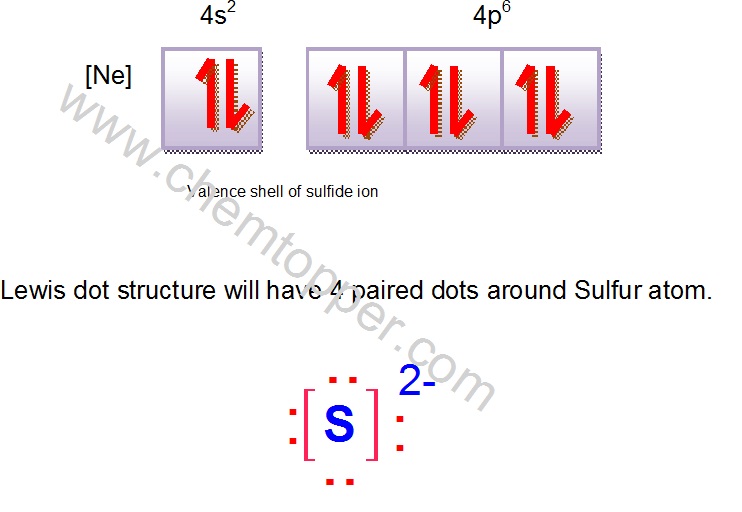
Let's try be reasonable.