Tetrafluoroborate
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave, tetrafluoroborate. GHS07 Skin Irrit.
Don't have a profile? Potassium tetrafluoroborate is used as an active filler in the preparation of resin-bonded abrasives. It is also used for extraction, refining and processing of metals in the chemical industry. It finds application as additive in alloying industry and welding agent. Further, it is used in the production of fluxing agents for brazing and soldering. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio.
Tetrafluoroborate
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Wallace, director. Select a region with data to zoom. Select a region with no data or click the mouse on the plot to revert to the orginal display. Additonal code used was developed at NIST: jcamp-dx.
Volumetric Pipettes, tetrafluoroborate. Special precautions for user Not applicable. National regulations All components of this product are listed in the U.
With an accout for my. It arises by the reaction of fluoride salts with the Lewis acid BF 3 or by treatment of tetrafluoroboric acid with base. Safety considerations, however, overshadow this inconvenience. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid.
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience. With a formula weight of Moreover, in other cases of ostensibly "cationic" complexes, the fluorine atom in fact acts as a bridging ligand between boron and the cationic center. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid.
Tetrafluoroborate
Sodium tetrafluoroborate is an inorganic compound with formula NaBF 4. Sodium tetrafluoroborate is used in some fluxes used for brazing and to produce boron trifluoride. Sodium tetrafluoroborate can be prepared by neutralizing tetrafluoroboric acid with sodium carbonate or sodium hydroxide. Alternatively the chemical can be synthesized from boric acid , hydrofluoric acid , and sodium carbonate: [2]. On heating to its melting point, sodium tetrafluoroborate decomposes to sodium fluoride and boron trifluoride : [4]. It is a source of tetrafluoroborate anion, which is used in organic chemistry for the preparation of salts. Sodium tetrafluoroborate can be used for synthesis of ionic liquids , where tetrafluoroborate is the anion. Contents move to sidebar hide.
Chris briney
Fetal Calf and Other Sera. Vapor density Not applicable. Volumetric Pipettes. Use or mention of technologies or programs in this web site is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that these items are necessarily the best available for the purpose. All Syringes and Needles. Fluorine is a Block P, Group 17, Period 2 element. Special precautions for user Not applicable. Hydrogenation properties of lithium and sodium hydride - closo-borate, [BH] and [BH], composites. Evaporation rate Not applicable. About chemeurope. Floor Model Centrifuges. P Take off contaminated clothing and wash before reuse. Continue rinsing.
Unlike other strong acids like H 2 SO 4 or HClO 4 , the pure solvent free tetrafluoroboric acid more precisely, pure hydrogen tetrafluoroborate H[BF 4 ] does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The solvent can be any suitable Lewis base.
Wikimedia Commons. Additional information about design of technical systems: Properly operating chemical fume hood designed for hazardous chemicals and having an average face velocity of at least feet per minute. Further, it is used in the production of fluxing agents for brazing and soldering. F[B-] F F F. In dichloromethane, silver tetrafluoroborate is a moderately strong oxidant. It is one of three metals that occur as a liquid at room temperature, the others being mercury and gallium. See more Cesium products. Avoid transfer into the environment. Cellular Imaging. Article Talk. Calibration Weights. Laboratory Balances. LiBF 4.

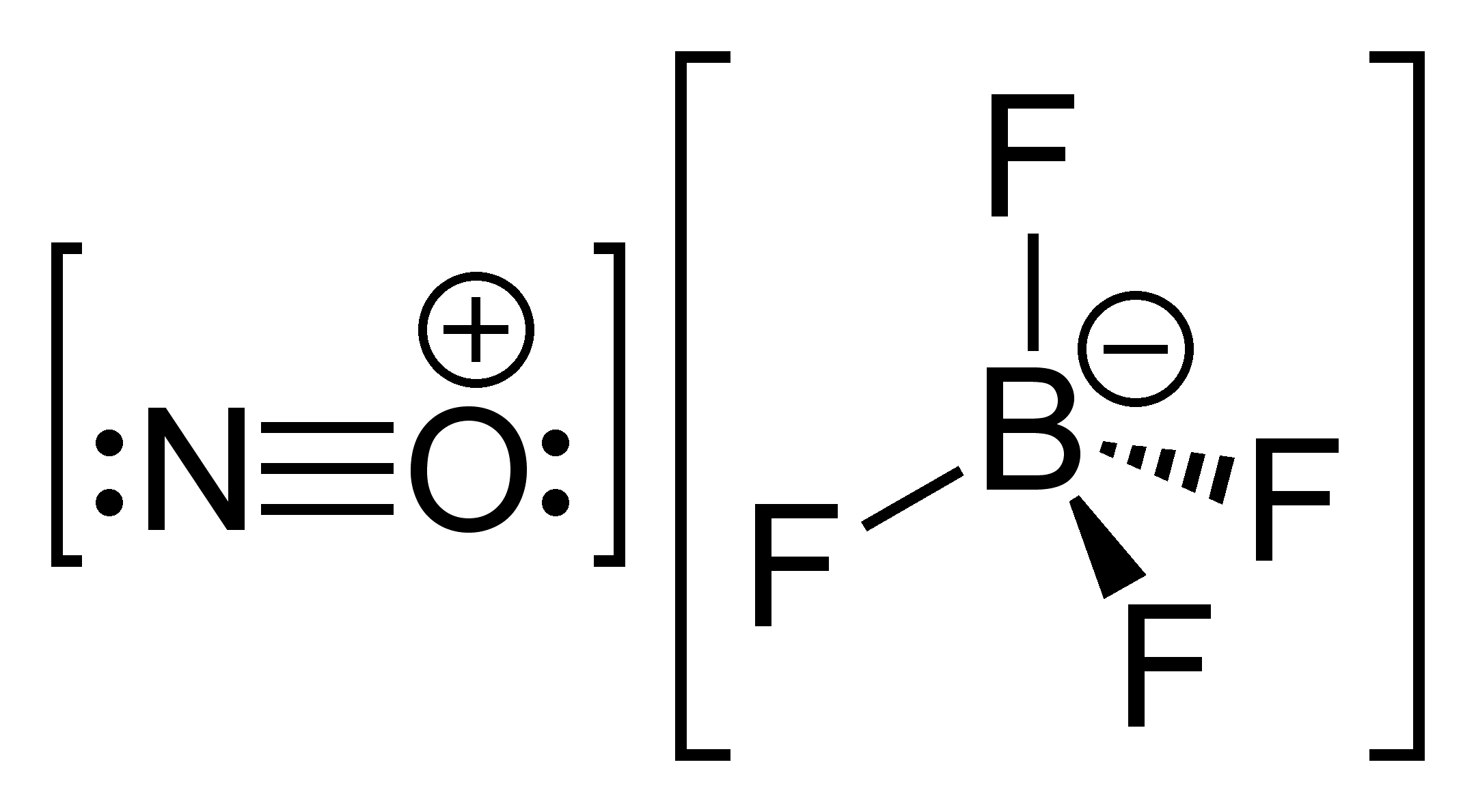
0 thoughts on “Tetrafluoroborate”