The isoelectronic pair is
The isoelectronic pair is. Halide ions often react with molecules of halogens or interhalogens to form polyhalide ions consisting either of the same halogen or of two or three different halogens. Besides these, a few othe anions are known, the isoelectronic pair is, which do not contain any of the halogen atoms but behave like halide ions. These anions are called pseudohalides and consist of two of more atoms of which one is always a nitrogen atom.
The isoelectronic pairs is :. The isoelectronic pair of ions is -. The isoelectronic pair of 32 electrons is. Which of the following pair s represent s the isoelectronic species? The group having isoelectronic species is Which of the following is isoelectronic pair? The group having isoelectronic species is.
The isoelectronic pair is
Isoelectronic refers to two ions or molecules having the same electronic structure and the same number of valence electrons. Last updated on Dec 20, Candidates must go through the NDA1 previous year's papers. Attempting the NDA1 mock tests is also essential. Get Started. English Hindi. This question was previously asked in. Attempt Online. Electric charges and coulomb's law Basic. Start Now.
Haryana Civil Services.
Courses for Kids. Free study material. Offline Centres. Talk to our experts Last updated date: 22nd Feb Study Material. Important Questions.
The observation that isoelectronic species are usually isostructural, first made by Penny and Southerland in , known as the isoelectronic principle Geoff. Table 1 shows an example of isostructural isoelectronic species periodic trends. All of these molecules are octahedral and isoelectronic within their periods. Search site Search Search. Go back to previous article. Sign in.
The isoelectronic pair is
Species such as atoms, molecules, or ions having the same number of electrons are called isoelectronic species. It is quite easy to identify isoelectronic species either by counting the total number of electrons or by writing electronic configuration. The electronic configuration of atoms or ions can be easily written but that of molecules is slightly difficult. Thus, I prefer to identify such species by counting electrons. I have tried to present simple way for the calculation of electrons in either atom, ions or molecules below. As stated above, these species have the same number of electrons, right? But these have different atomic number different number of proton.
Kingdom hearts nobody tattoo
CG Vyapam SI. CISF Constable. Bihar Police Fire Station Officer. Civil Services Exam. The disease caused by breathing polluted air is:. MP Jail Prahari. UP Police Sub Inspector. Bihar Police SI. UIIC Assistant. FCI Stenographer. AAI Junior Assistant. Maharashtra Arogya Vibhag Group D. Trusted by 3. Which of the following is a polar compound? Give reason in one or two sentences form the following: 'o-nitrophenol
Two or more atoms, molecules, or ions that have the same number of electrons and electronic structure are known to be isoelectronic. Isoelectronic species typically have the same chemical properties.
Trending Questions. You can reuse this answer Creative Commons License. Odisha Assistant Agriculture Officer. Valence isoelectronic is another name for this criterion. Suggested Test Series. Eligibility Criteria. The major product of the reaction of 2-butene with cold alkaline KMnO Bihar LRC Clerk. Which of the following species have maximum number of unpaired elect Explanation: Isoelectronic means "same electronic structure".

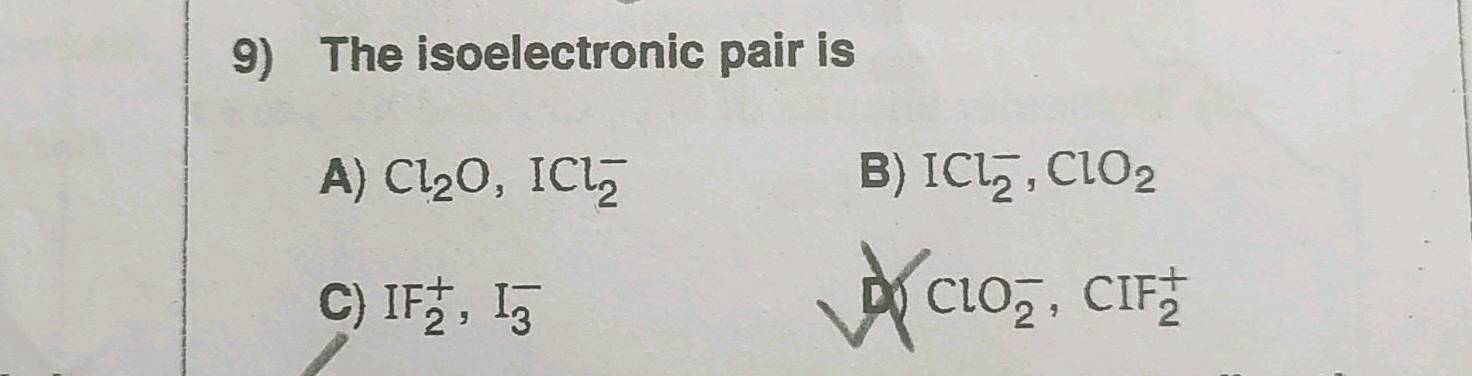
0 thoughts on “The isoelectronic pair is”