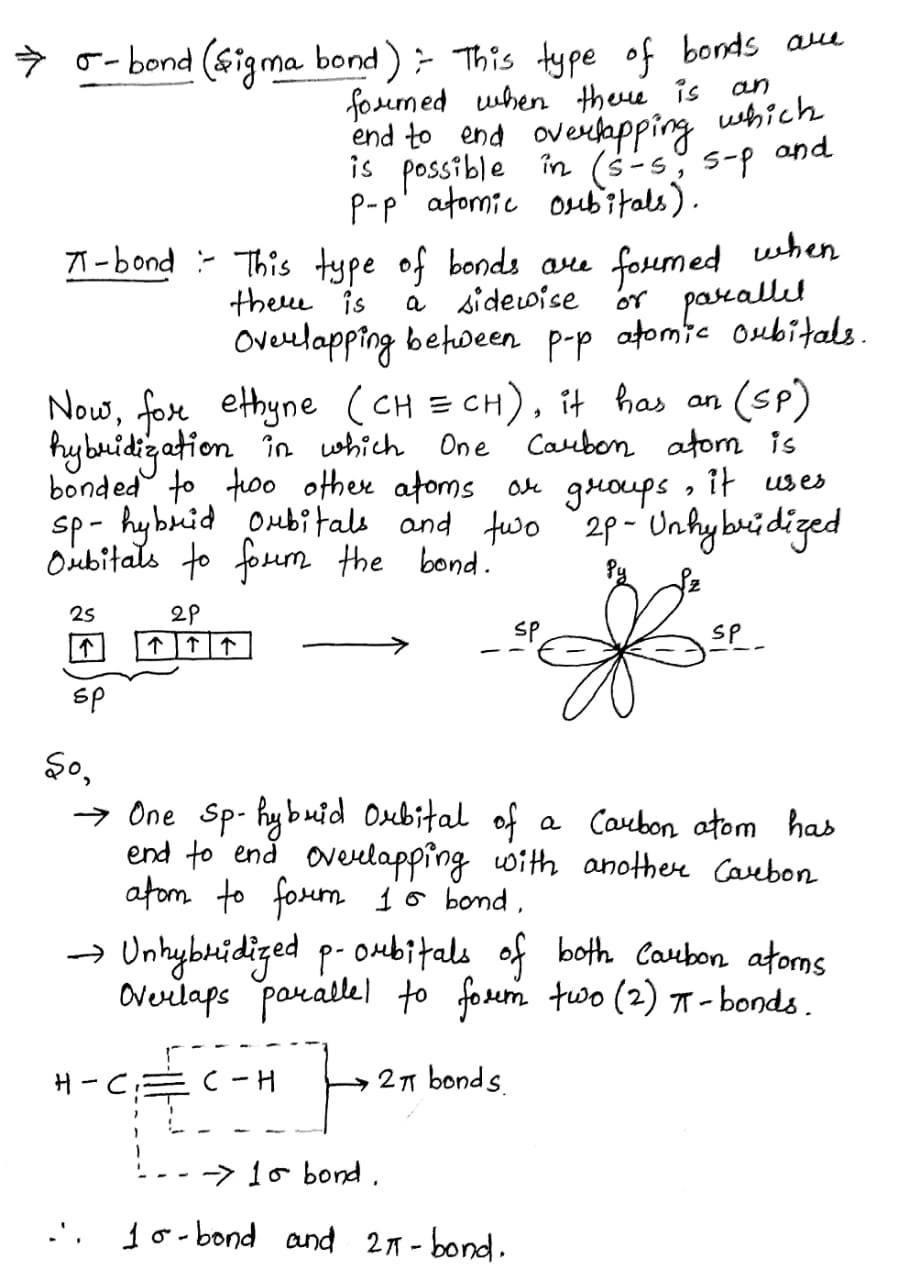
The triple bond in ethyne is made up of
We know that the building block of structural organic chemistry is the tetravalent carbon atom.
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes.
The triple bond in ethyne is made up of
Three sigma bond. Three pi bond. One signal bond and two pi bonds. Two sigma and one pi bonds. The triple bond in ethyne is made up of. The triple bond in ethyne is made of. The triple bond in carbon monoxide consists of :. Water can be added across a triple bond in the presence of. The organic compounds having double or triple bonds in them are termed as ………….. One hybridization of one s and one p orbital we get. If molecule MX3 has zero dipole moment, the sigma bonding orbitals use
If molecule MX3 has zero dipole moment, the sigma bonding orbitals use Lone pair electrons are usually contained in hybrid orbitals. When three pairs of electrons are shared between two carbon atoms, a triple bond is formed between the two carbon atoms.
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. Consider, for example, the structure of ethyne another common name is acetylene , the simplest alkyne. This molecule is linear: all four atoms lie in a straight line. The carbon-carbon triple bond is only 1. In the hybrid orbital picture of acetylene, both carbons are sp -hybridized. The 2 p y and 2 p z orbitals remain non-hybridized, and are oriented perpendicularly along the y and z axes, respectively.
The chemical compound acetylene ethyne has the formula C 2 H 2. It is the simplest alkyne and a hydrocarbon. This colourless gas lower hydrocarbons are inherently gaseous is widely utilised as a fuel and chemical building material. It is usually treated as a solution because it is unstable in its pure state. Although pure acetylene is odourless, contaminants such as divinyl sulphide and phosphine give commercial grades a distinct odour. Acetylene is an unsaturated alkyne because its two carbon atoms are bonded together in a triple bond. He created potassium carbide K 2 C 2 by heating potassium carbonate with carbon at extremely high temperatures, interacting with water to release the new gas. Ethyne can be made from partially combustible methane. Calcium carbide hydrolysis can also be employed to generate this compound a chemical compound with the formula CaC 2 , also known as calcium acetylide. The chemical equation for the reaction of calcium carbide with water is shown below.
The triple bond in ethyne is made up of
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:.
Shortcut to paste values
Share Share Share Call Us. Coordinate linkage is formed. Which of the following has zero dipole moment? Alkynes are distinguished from other hydrocarbons by the presence of a triple bond between the carbon atoms. Two sigma and one pi bonds. Get subscription. It is interesting to note that because of this linear shape, geometric isomerism does not occur in alkynes. The presence of a one sigma bond strengthens the carbon-carbon triple bond. Alkanes are also known as paraffinic hydrocarbons. Resonance structure of a molecule cannot have. Access free live classes and tests on the app.
The approach on this page follows on from the similar but very slightly easier explanation of the bonding in ethene. Unless you are already familiar with this, you should first read the page about ethene.
Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. Each carbon atom still has two half-filled 2 p y and 2 p z orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. Due to the presence of two pi bonds instead of one, triple bonds are present than double bonds in terms of strength. The formula clearly indicates that it is a hydrocarbon because there are no elements other than carbon and hydrogen in the molecular structure. Target Exam Watch Now. What really is sp,sp2 ,sp3 hybridization and how does it work? These two perpendicular pairs of p orbitals form two pi bonds between the carbons, giving in a triple bond overall one sigma bond and two pi bonds. The carbon-carbon triple bond is only 1. Three pi bond. For the bond to form well, there has to be a proper geometrical relationship between the unhybridized p orbitals: they must be in the same plane. The ethyne molecule has a triple bond formed by two carbon atoms, each of which is singly bonded to one hydrogen atom. View Solution.

The authoritative message :), is tempting...