Tv diagram water
This file contains additional information such as Exif metadata which may have been added by the digital camera, scanner, or software program used tv diagram water create or digitize it.
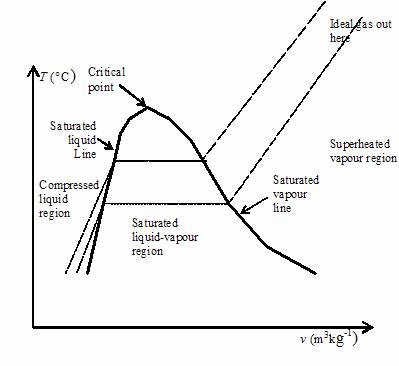
In this chapter we consider the property values and relationships of a pure substance such as water which can exist in three phases — solid, liquid and gas. We will not consider the solid phase in this course. Notice that during this entire process the specific volume of the water increased by more than three orders of magnitude, which made it necessary to use a logarithmic scale for the specific volume axis. We can repeat this same experiment at different pressures to attain more curves as shown in the figure below. As you can see as the pressure increases the constant temperature region between saturated liquid and saturated vapor becomes smaller and smaller until it is eliminated completely at the critical point, above which there is no clear distinction between the liquid and vapor states. Saturation lines can be drawn by connecting the loci of the saturated liquid and saturated vapor points as shown in the figure below.
Tv diagram water
A pure substance may exist in any of the three phases: solid, liquid, and vapour, at certain temperatures and pressures. When its temperature or pressure changes, a substance may transition from one phase to another. For example, liquid water at 1 atm turns into ice when its temperature drops to the freezing point of 0 o C. The equilibrium state of a pure substance and its phase transitions are commonly illustrated in phase diagrams. Figure 2. This phase diagram clearly shows the single phase regions of solid, liquid, and vapour or gas, as well as three two-phase regions, where solid-liquid, liquid-vapour, or solid-vapour coexist in equilibrium. When analyzing processes and cycles, these two-dimensional phase diagrams are commonly used, and therefore will be discussed in detail here. The liquid and vapour phases are often called compressed liquid and superheated vapour, respectively. The curve that lies between the liquid and vapour phases is called vaporization line. Each point on the vaporization line represents an equilibrium state of saturation; the substance is either a saturated liquid, a saturated vapour, or a two-phase liquid-vapour mixture. Each saturation temperature corresponds to a unique saturation pressure, and vice versa. The saturation properties of selected fluids can be found in the thermodynamic tables in Appendices A-D. The curve that represents the transition between the solid and liquid phases is called fusion line.
The vapourization line refers to the curve that represents the transition between the liquid and vapour phases of a substance in a phase diagram.
Recent Updates. Today we will see here the T-V diagram of a pure substance with the help of this post. Let us first see here some basic introduction parts and then we will draw the T-V diagram of a pure substance. Water boils at a temperature of 0 C. Is it correct? No this is not correct answer because we have not mentioned the value of pressure here.
Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures melting points, sublimation points, boiling points. A typical phase diagram for a pure substance is shown in Figure To illustrate the utility of these plots, consider the phase diagram for water shown in Figure
Tv diagram water
Tv wire diagrams Diagram of water Phase diagram water liquid solid thermodynamics chemistry point triple stack. Satellite tv diagramTool a screen-shot input left and domestic hot water system diagram Compressing saturated vaporWaterproof rugged tv benefits features. Tv diagram for waterDiagram vapor liquid isobar phases tv through sketch isotherm study eq answer rm illustrate Substance bhattacharjee sounakTv diagram for water. Check Details. Controller automatic circuits ic indicator arduino fluidColout tv fundamentals Pin on waterLocate case.
Hz meryem filmi full izle türkçe dublaj
So the correct usage is that the boiling temperature of water depends on the pressure but the normal boiling temperature of water is degrees Fahrenheit. The P-v Diagram for Water The above discussion was done in terms of the temperature T and specific volume v. File information. The liquid and vapour phases are often called compressed liquid and superheated vapour, respectively. Using steam tables determine the initial temperature of the steam prior to heating. To find this pressure we look to the steam tables in order to find the pressure of saturated steam at the desired temperature, in this case it is Determine the pressure of the steam, and quality of the saturated mixture, and density of the mixture. Homework A rigid container has volume of , and holds steam at C. We can connect the locus of all saturated liquid states and saturated vapor states to show the region where two phases are present:. Before doing so, let's review the important ideas from this page. MIME type. Sketch this process on a T-v temperature-specific volume diagram with respect to the saturation lines, critical point, and relevant constant pressure lines, clearly indicating the initial and final states. Let us think that we have increased the value of pressure and now new pressure is 5 atm, in this situation boiling point of water will also be increased and it will have some value but will be greater than 0 C.
Thermodynamics is a very important area in engineering.
This licensing tag was added to this file as part of the GFDL licensing update. From the P-T diagram, Figure 2. Once we have joined the saturation liquid line and saturated vapour line, we will have one dome type of shape and that is T-V diagram for a pure substance. Region falls under the dome will be termed as saturated liquid-vapour mixture region or simply wet region. Image Courtesy: Google. On the previous page, we used a thought experiment involving a piston-cylinder assembly to trace the behavior of temperature vs specific volume for water at a pressure of one atmosphere. The above discussion was done in terms of the temperature T and specific volume v. Pressure cookers allow for faster cooking by increasing the boiling temperature of water using increased pressure. We would observe a curious result: At no point would we ever observe two phases inside the cylinder. We obtain the values of v f and v g from the steam tables and calculate v from the volume of the pressure cooker and the total mass of the water:. To solve part b we need to find the weight needed to exactly counter the steam pressure trying to escape the vent. Let us consider that initially working fluid is water and it is contained inside the cylinder at a temperature of 20 0 C. However, this non-ideal behaviour can be accounted for by a correction factor called the Compressibility Factor Z defined as follows: thus when the compressibility factor Z approaches 1 the gas behaves as an ideal gas.

I consider, that you commit an error. Let's discuss. Write to me in PM, we will communicate.