Ug l in ppm
We have included the following conversion tables to further your experience at Water on the Web.
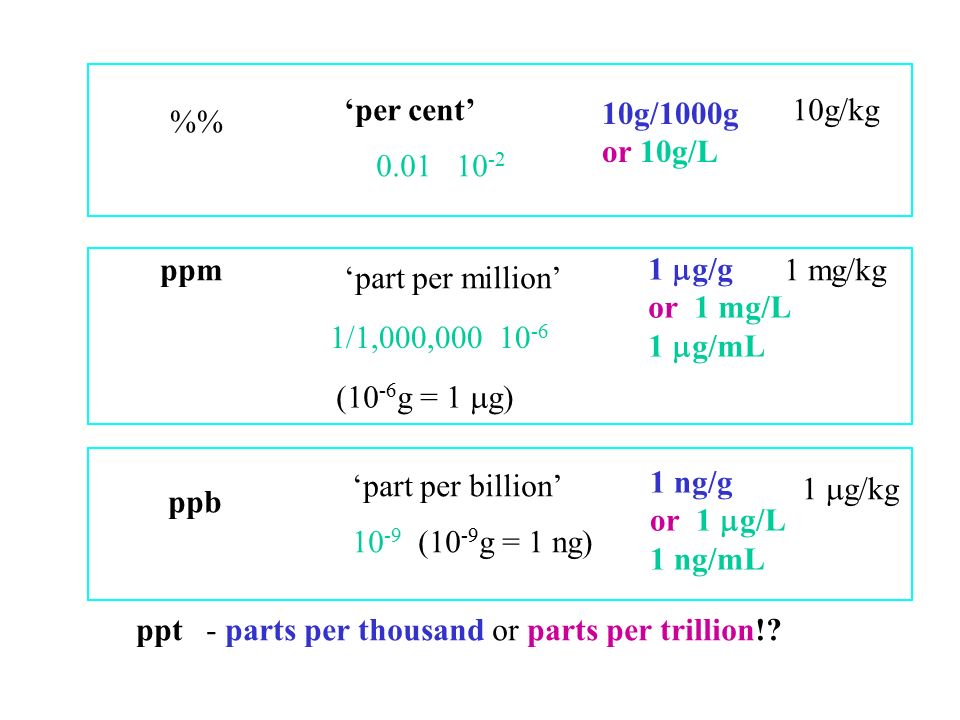
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution. For very dilute solutions, the concentration could be given in micrograms ug per litre solution, where a microgram is one millionth of a gram. Parts per million ppm is another common way to describe concentration. It stands for the number of "parts" of chemical such as grams per parts also grams of total solution. You can convert between these two types of measurement by using the density of the solution.
Ug l in ppm
The unit ppm is used in several branches in different ways. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. For more theory about the use of ppm, please see the documentation below. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. If the molecular weight is unknown to you, please try our Molecular Weight Calculator. The significance is automatically determined. Use extra zero's to expand the significance. In air pollution literature ppm applied to a gas, always means parts per million by volume or by mole. These are identical for an ideal gas, and practically identical for most gases of air pollution interest at 1 atm. Another way of expressing this value is ppmv.
Kinematic Viscosity Calculator. PPM to Molarity Convert ppm to gram based or milligram based concentration.
PPM conversion values and serial dilutions : Ppm concentrations and percentages. Ppm to Molarity and Molarity to ppm. Signature: Dhanlal De Lloyd, Chem. Augustine campus The Republic of Trinidad and Tobago. One gram in ml is ppm and one thousandth of a gram 0.
To switch between the two conversions, simply use the swap icon rotating arrows. If you need to start over, you can reset the values by clicking the reset button. Article Contents [ show ]. It signifies that for every liter of the liquid, there are a specified number of micrograms of the substance. Now, let's break down this seemingly complex term:.
Ug l in ppm
The unit ppm is used in several branches in different ways. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. For more theory about the use of ppm, please see the documentation below.
Euro kac tl
And from this 10 -4 M soln. Concentration Analogies: Explaining chemical concentrations parts per million, parts per billion by using analogies from : Michael A. Osmotic pressure calculator. Mass of Ca to get Molarity. This law implies that 1 mole of gas at STP a volume of Most of the chemical data that is reported for waterbodies is expressed as a concentration: a mass of chemical per unit volume of water. These are identical for an ideal gas, and practically identical for most gases of air pollution interest at 1 atm. About Lenntech. FAQs tutorials glossary conversion tables links. Hence, weigh out 2. The density of pure water has to be by definition One Part Per Quadrillion one postage stamp on a letter the size of California and Oregon one human hair out of all the hair on all the heads of all the people in the world one mile on a journey of light years. Pipette 10 ml of the 1M stock into a ml volumetric flask and make up to the mark to give a 10 -1 M soln. Hardness Converter and Hardness Calculator. Hence, weigh out 1.
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution.
The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. Taking the Risk out of Reporting Risk Assessment. PPM to Molarity Convert ppm to gram based or milligram based concentration. Mass of Ca to get Molarity. Accuracy water analysis calculation. Comparable Water Volume. Because a milligram is equal to micrograms and a kilogram is equal to grams, this change in units does not change the actual value of the number because the ratio of the units has not changed. You now have your solution concentration in units of milligrams per kilograms, which is the same as parts per million. Osmotic pressure calculator. In the case of the example, if the solution density was 1. This is also equivalent to 32, ppm part-per-million. Katz and Martha L. Augustine campus The Republic of Trinidad and Tobago.

In my opinion you are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.