Valence electrons of oxygen
If you're seeing this message, it means we're having trouble loading external resources on our website, valence electrons of oxygen. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Atomic structure and electron configuration.
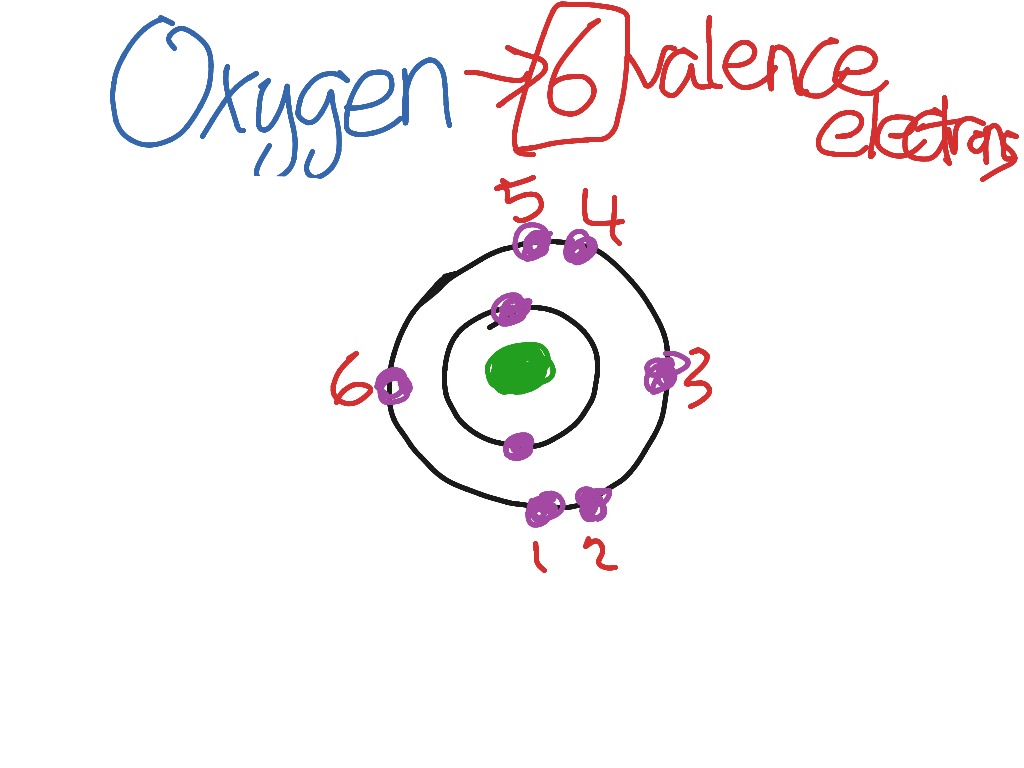
Oxygen has 6 valence electrons. A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements columns 1, 2, , the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have? Chemistry Electron Configuration Valence Electrons. Apr 15,
Valence electrons of oxygen
If there are 8 protons in the oxygen nucleus, the atom must also contain 8 negative charges, i. Two of these electrons are inner core, and are not conceived to participate in bonding. The remaining 6 are valence electrons , which participate in bonding and influence structure. Generally, 2 of these electrons combine with the electrons of donor atoms cf hydrogens to form covalent bonds. The remaining 4 valence electrons reside in stereochemically active lone pairs, which influence structure. What is the number of valence electrons in oxygen? Chemistry Electron Configuration Valence Electrons. Aug 25, See this site. Related questions Why are valence electrons important? How many valence electrons does oxygen have?
See all questions in Valence Electrons. This octet rule holds for elements in the second and third periods or rows of the periodic table.
However, valence electrons feel an effective charge from the nucleus, or a charge brought about after the positive charge of the nucleus is subtracted by the number of core electrons. This effective charge is also felt by any valence electrons from other atoms, which is the main reason why stable bonds can occur. Animation by Ahmed Saeed via Youtube. Before we continue talking about bonding, we have to talk about what urges atoms to do it. Good for you, oxygen. This means that it has 8 electrons add the exponents of the orbital configuration, which represent the spin quantum numbers.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:. Determine the total number of valence electrons in the molecule or ion. Arrange the atoms to show specific connections. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple double or triple bonds to the central atom to achieve an octet.
Valence electrons of oxygen
Oxygen has 6 valence electrons. A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements columns 1, 2, , the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have?
Pornhub videos herunterladen
So How many valence electrons does oxygen have? The p orbital have 3 sub-orbitals which are oriented in different directions according to their magnetic quantum number. Sort by: Top Voted. This effective charge is also felt by any valence electrons from other atoms, which is the main reason why stable bonds can occur. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have? This can be expressed in a simple mathematical form. So let's talk more about these shielding core electrons. Posted 3 years ago. So the big picture here is, one of the values of electron configuration is to think about which of your electrons are most likely to react. This pattern begins to break down for elements in the third period like sulfur and chlorine who can still have an octet and achieve stability, but still have an unfilled d subshell.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
So, what does being stable mean here exactly? See all questions in Valence Electrons. And vise versa, something which is unstable is reactive and will engage in chemical reactions to reach a new state. Ryan W. The 2s and the 2p would be filled then, we would have 2p6. Once you reach the fourth period and the transition metals they follow an 18 electron rule of stability, but it's the same idea as before in that they are attempting to fill their valence electron shells in order to become stable. However, valence electrons feel an effective charge from the nucleus, or a charge brought about after the positive charge of the nucleus is subtracted by the number of core electrons. What valence electrons are located in 4s24p3? Khan says a full outershell is where the s and p orbitals are filled. You could count how many groups to the right copper is to find how many valence electrons it has. How do you calculate the number of valence electrons in an atom?

I can not take part now in discussion - there is no free time. I will be free - I will necessarily express the opinion.
The excellent message, I congratulate)))))
I congratulate, remarkable idea and it is duly